.
Life History of the Kangaroo Rat, Charles T. Vorhies and Walter P. Taylor
UNITED STATES DEPARTMENT OF AGRICULTURE
BULLETIN No. 1091
Also Technical Bulletin No. 1 of the Agricultural Experiment Station University of Arizona
| Washington, D. C. | PROFESSIONAL PAPER | September 13, 1922 |
LIFE HISTORY OF THE KANGAROO RAT
Dipodomys spectabilis spectabilis Merriam
BY
CHARLES T. VORHIES, Entomologist
Agricultural Experiment Station, University of Arizona; and
WALTER P. TAYLOR, Assistant Biologist
Bureau of Biological Survey, U. S. Department of Agriculture
CONTENTS
| Importance of Rodent Groups | 1 |
| Identification | 3 |
| Description | 5 |
| Occurrence | 7 |
| Habits | 9 |
| Food and Storage | 18 |
| Burrow Systems, or Dens | 28 |
| Commensals and Enemies | 33 |
| Abundance | 36 |
| Economic Considerations | 36 |
| Summary | 38 |
| Bibliography | 40 |

WASHINGTON
GOVERNMENT PRINTING OFFICE
1922
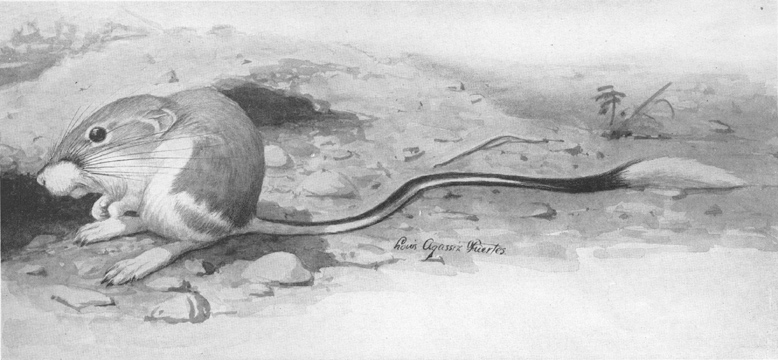 Plate I.—Banner-tailed Kangaroo Rat (Dipodomys spectabilis spectabilis Merriam).
Plate I.—Banner-tailed Kangaroo Rat (Dipodomys spectabilis spectabilis Merriam).From Dipodomys merriami Mearns and subspecies, which occur over much of its range, this form is easily distinguished by its larger size and the conspicuous white brush on the tail.
 |
UNITED STATES DEPARTMENT OF AGRICULTUREBULLETIN No. 1091Also Technical Bulletin No. 1 of the Agricultural Experiment Station, University of Arizona |
 |
| Washington, D. C. | PROFESSIONAL PAPER | September, 1922 |
LIFE HISTORY OF THE KANGAROO RAT,
Dipodomys spectabilis spectabilis Merriam.
By Charles T. Vorhies, Entomologist, Agricultural Experiment Station, University of Arizona; and Walter P. Taylor, Assistant Biologist, Bureau of Biological Survey, U. S. Department of Agriculture.
CONTENTS.
| Page | |
| Importance of rodent groups | 1 |
| Investigational methods | 2 |
| Identification | 3 |
| Description | 5 |
| General characters | 5 |
| Color | 6 |
| Oil gland | 6 |
| Measurements and weights | 7 |
| Occurrence | 7 |
| General distribution | 7 |
| Habitat | 7 |
| Habits | 9 |
| Evidence of presence | 9 |
| Mounds | 9 |
| Runways and tracks | 10 |
| Signals | 11 |
| Voice | 12 |
| Daily and seasonal activity | 12 |
| Pugnacity and sociability | 13 |
| Sense developments | 14 |
| Movements and attitudes | 15 |
| Storing habits | 15 |
| Breeding habits | 16 |
| Food and storage | 18 |
| Burrow systems, or dens | 28 |
| Commensals and enemies | 33 |
| Commensals | 33 |
| Natural checks | 34 |
| Parasites | 35 |
| Abundance | 36 |
| Economic considerations | 36 |
| Control | 37 |
| Summary | 38 |
| Bibliography | 40 |
Note.—This bulletin, a joint contribution of the Bureau of Biological Survey and the Arizona Agricultural Experiment Station, contains a summary of the results of investigations of the relation of a subspecies of kangaroo rat to the carrying capacity of the open ranges, being one phase of a general study of the life histories of rodent groups as they affect agriculture, forestry, and grazing.
IMPORTANCE OF RODENT GROUPS.
As the serious character of the depredations by harmful rodents is recognized, State, Federal, and private expenditures for their control increase year by year. These depredations include not only the attacks by introduced rats and mice on food materials stored in granaries, warehouses, commercial establishments, docks, and private houses, but also, particularly in the Western States, the ravages of several groups of native ground squirrels and other noxious rodents in grain and certain other field crops. Nor is this all, for it has [2]been found that such rodents as prairie dogs, pocket gophers, marmots, ground squirrels, and rabbits take appreciable and serious toll of the forage on the open grazing range; in fact, that they reduce the carrying capacity of the range to such an extent that expenditures for control measures are amply justified. Current estimates place the loss of goods due to rats and mice in warehouses and stores throughout the United States at no less than $200,000,000 annually, and damage to the carrying capacity of the open range and to cultivated crops generally by native rodents in the Western States at $300,000,000 additional; added together, we have an impressive total from depredations of rodents.
The distribution and life habits of rodents and the general consideration of their relation to agriculture, forestry, and grazing, with special reference to the carrying capacity of stock ranges, is a subject that has received attention for many years from the Biological Survey of the United States Department of Agriculture. As a result of the investigations conducted much has been learned concerning the economic status of most of the more important groups, and the knowledge already gained forms the basis of the extensive rodent-control work already in progress, and in which many States are cooperating with the bureau. If the work is to be prosecuted intelligently and the fullest measure of success achieved, it is essential that the consideration largely of groups as a whole be supplemented by more exhaustive treatment of the life histories of individual species and of their place in the biological complex. The present report is based upon investigations, chiefly in Arizona, of the life history, habits, and economic status of the banner-tailed kangaroo rat, Dipodomys spectabilis spectabilis Merriam (Pl. I).
INVESTIGATIONAL METHODS.
Some 18 years ago (in 1903) a tract of land 49.2 square miles in area on the Coronado National Forest near the Santa Rita Mountains, Pima County, southern Arizona, was closed to grazing by arrangement between the Forest Service and the Agricultural Experiment Station of the University of Arizona. Since that time another small tract of nearly a section has been inclosed (Griffiths, 1910, 7[1]). This total area of approximately 50 square miles is known as the United States Range Reserve, and is being devoted to a study of grazing conditions in this section and to working out the best methods of administering the range (Pl. II, Fig. 1).
[3]For some years an intensive study of the forage and other vegetative conditions of this area has been made, the permanent vegetation quadrat, as proposed by Dr. F. E. Clements (1905, 161-175), being largely utilized. During the autumn of 1917 representatives of the Carnegie Institution and the Arizona Agricultural Experiment Station visited the Reserve and were impressed with the evidence of rodent damage to the grass cover. The most conspicuous appearance of damage was noted about the habitations of the banner-tailed kangaroo rat (Dipodomys spectabilis spectabilis Merriam), although it was observed also that jack rabbits of two species (Lepus californicus eremicus Allen and L. alleni alleni Mearns), which were very abundant in some portions of the reserve, were apparently affecting adversely the forage conditions in particular localities. Accordingly, the Biological Survey, the Agricultural Experiment Station of the University of Arizona, the Carnegie Institution of Washington, and the U. S. Forest Service have undertaken a study of the relation of the more important rodents to the forage crop of the Range Reserve in Arizona.
The present paper is a first step in this larger investigation.[2] In this work the authors have made no attempt to deal with the taxonomic side of the kangaroo rat problem. It is not unlikely that intensive studies will show that the form now known as Dipodomys spectabilis spectabilis is made up of a number of local variants, some of them perhaps worthy of recognition as additional subspecies. But it is felt that the conclusions here reached will be little, if at all, affected by such developments.
Color descriptions are based on Ridgway's Color Standards and Color Nomenclature published in 1912.
IDENTIFICATION.
There are only three groups of mammals in the Southwest having external cheek pouches. These are (a) the pocket gophers (Geomyidæ), which have strong fore feet, relatively weak hind feet, and short tail, as compared with weak fore feet, relatively strong hind feet, and long tail in the other two; (b) the pocket mice (Perognathus), which are considerably smaller than the kangaroo rats and[4] lack the conspicuous white hip stripe possessed by all the latter; and (c) the kangaroo rats (Dipodomys).
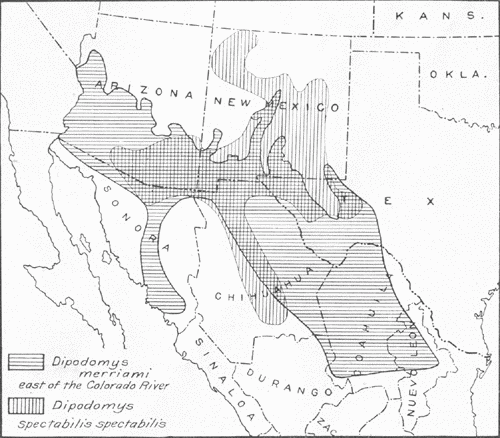 Fig. 1.—Range, east of the Colorado River, of Dipodomys spectabilis spectabilis compared with that of Dipodomys merriami. Cross hatching indicates area of overlapping of the two forms. The range of Dipodomys deserti, not shown on the map, is west of that of spectabilis, and so far as known the two do not overlap.
Fig. 1.—Range, east of the Colorado River, of Dipodomys spectabilis spectabilis compared with that of Dipodomys merriami. Cross hatching indicates area of overlapping of the two forms. The range of Dipodomys deserti, not shown on the map, is west of that of spectabilis, and so far as known the two do not overlap.Dipodomys spectabilis spectabilis Merriam requires comparison with three other forms of kangaroo rats in the same general region, namely, D. deserti Stephens, of approximately the same size, and D. merriami Mearns and D. ordii Woodhouse, the last two of decidedly smaller size. The range of deserti lies principally to the west of that of spectabilis, and the two do not, so far as known, overlap. On the other hand, merriami and ordii, and subspecies, occur over a large part of the range of spectabilis, living in very close proximity to its burrows; merriami is even suspected of pillaging the stores of spectabilis. The range of merriami, however, is much more extensive than that of spectabilis (Fig. 1), which argues against a definite ecological dependence or relationship. Separation of the four forms mentioned may be easily accomplished by the following key:
[5]Key to Species of Dipodomys in Arizona.
a1. Size much larger (hind foot and greatest length of skull more than 42 millimeters); tail tipped with white.
b1. Upper parts dark brownish buffy; tail dark brownish or blackish with more sharply contrasted white tip; interparietal broader, distinctly separating mastoids (range in Arizona mainly southeastern part)Dipodomys spectabilis.
b2. Upper parts light ochraceous-buffy; tail pale brownish with less sharply contrasted white tip; interparietal narrower, reduced to mere spicule between mastoids (range in Arizona mainly southwestern part)) Dipodomys deserti.
a2. Size much smaller (hind foot and greatest length of skull less than 42 millimeters); tail not tipped with white.
b1. Hind foot with four toes
Dipodomys merriami.
b2. Hind foot with five toes
Dipodomys ordii.
On account of the small size, merriami and ordii do not require detailed color comparison with the other two. The general color of the upperparts of spectabilis is much darker than that of deserti; whereas spectabilis is ochraceous-buff or light ochraceous-buff grizzled with blackish, deserti is near pale ochraceous-buff and lacks the blackish.
The color of the upperparts alone amply suffices to distinguish spectabilis and deserti; but the different coloration of the tail is the most obvious diagnostic feature. The near black of the middle portion of the tail, the conspicuous white side stripes, and the pure white tip make the tail of spectabilis stand in rather vivid contrast to the pale-brown and whitish tail of deserti.
The dens of the two larger species of Dipodomys—spectabilis and deserti—can be distinguished at a glance from those of the two smaller—merriami and ordii—by the fact that the mounds of the former are usually of considerable size and the burrow mouths are of greater diameter. On the Range Reserve merriami erects no mounds, but excavates its burrows in the open or at the base of Prosopis, Lycium, or other brush. The mounds of spectabilis are higher than those of deserti, the entrances are larger, and they are located in harder soil (Pl. III, Fig. 1). The dens of deserti are usually more extensive in surface area than those of spectabilis, and have a greater number of openings (Pl. III, Fig. 2).
 Plate II. Fig. 1.—Winter View of Area Inhabited by Kangaroo Rats.
Plate II. Fig. 1.—Winter View of Area Inhabited by Kangaroo Rats.A water-hole scene on the U. S. Range Reserve at the base of the Santa Rita Mountains, Ariz., where cooperative investigations are being conducted to ascertain the relation of rodents to forage. This is typical of a large section of country occupied by Dipodomys spectabilis spectabilis and Dipodomys merriami. The brush is mesquite (Prosopis), cat's-claw (Acacia), and paloverde (Cercidium).
 Plate II. Fig. 2.—Kangaroo Rat Country Following Summer Drought.
Plate II. Fig. 2.—Kangaroo Rat Country Following Summer Drought.An area of the U. S. Range Reserve in the autumn of 1918, showing the result of failure of summer rains. Such a condition is critical not only for the stockmen but also for kangaroo rats and other desert rodents, and results in competition between them as to which shall benefit by what the range has to offer.
 Plate III. Fig. 1.—Kangaroo Rat Mound (Dipodomys s. spectabilis).
Plate III. Fig. 1.—Kangaroo Rat Mound (Dipodomys s. spectabilis).Typical Dipodomys s. spectabilis mound on the Range Reserve, under shelter of desert hackberry (Celtis pallida). Most dens on the reserve are located in the shelter of brush plants, the more important being mesquite (Prosopis velutina), cat's-claw (Acacia spp.), and the desert hackberry. (See also Pl. VIII Fig. 2.)
 Plate III. Fig. 2.—Kangaroo Rat Mound (Dipodomys deserti).
Plate III. Fig. 2.—Kangaroo Rat Mound (Dipodomys deserti).Den of Dipodomys deserti deserti, showing typical wide, low mound with numerous entrance holes. This species excavates its den in soft, sandy soil. The tree is a species of Dalea.
DESCRIPTION.
GENERAL CHARACTERS.
Size large; ears moderate, ear from crown (taken in dry skin) 9 or 10 millimeters; eyes prominent; whiskers long and sensitive; fore feet short and weak; hind feet long and powerful, provided with four well-developed toes; tail very long, usually 30 to 40 per cent[6] longer than the body. Cranium triangular, the occiput forming the base and the point of the nose the apex of the triangle, much flattened, auditory and particularly mastoid bullae conspicuously inflated.
COLOR.
General color above, brownish buffy, varying in some specimens to lighter buffy tints, grizzled with black; oblique hip stripes white; tail with dark-brown or blackish stripes above and below, running into blackish about halfway between base and tip, and with two lateral side stripes of white to a point about halfway back; tail tipped with pure white for about 40 millimeters (Pl. I). Underparts white, hairs white to bases, with some plumbeous and buffy hairs about base of tail; fore legs and fore feet white all around; hind legs like back, brown above, hairs with gray bases, becoming blackish (fuscous-black or chætura-black) about ankles, hairs on under side white to bases; hind feet white above, dark-brown or blackish (near fuscous) below.
Color variations in a series of 12 specimens from the type locality and points widely scattered through the range of spectabilis consist in minor modifications of the degree of coloration, length of white tip of tail, and length of white lateral tail stripes. In general the color pattern and characters are remarkably uniform. Young specimens, while exhibiting the color pattern and general color of adults, are conspicuously less brown, and more grayish.
There appears to be little variation in color with season. In the series at hand, most specimens taken during the fall, winter, and spring are very slightly browner than those of summer, suggesting that the fresh pelage following the fall molt is a little brighter than is the pelage after being worn all winter and into the following summer. But at most the difference is slight.
OIL GLAND.
Upon separating the hairs of the middle region of the back about a third of the distance between the ears and the rump, one uncovers a prominent gland, elliptical in outline, with long axis longitudinal and about 9 millimeters in length. The gland presents a roughened and granular appearance, and fewer hairs grow upon it than elsewhere on the back. The hairs in the vicinity are frequently matted, as if with a secretion. In worn stage of pelage the gland may be visible from above without separating the hairs. Bailey has suggested that this functions as an oil gland for dressing the fur, and our observations bear out this view. Kangaroo rats kept in captivity without earth or sand soon come to have a bedraggled appearance, as if the pelage were moist. When supplied with fine,[7] dusty sand, they soon recover their normal sleek appearance. Apparently the former condition is due to an excess of oil, the latter to the absorption of the excess in a dust bath. The oil is doubtless an important adjunct to the preservation of the skin and hair amid the dusty surroundings in which the animal lives.
MEASUREMENTS AND WEIGHTS.
External measurements include: Total length, from tip of nose to tip of tail without hairs, measured before skinning; tail vertebræ, length of tail from point in angle when tail is bent at right angles to body to tip of tail without hairs; and hind foot, from heel to tip of longest claw.
The following are measurements of a series from the U. S. Range Reserve:
Average measurements of 30 adult specimens of both sexes: Total length, 326.2 millimeters (349-310); tail vertebræ, 188.4 (208-180); hind foot, 49.5 (51-47); the average weight of 29 adult specimens of both sexes was 114.5 grams (131.9-98.0).
Averages for 17 adult females: Total length, 326.4 millimeters (349-310); tail vertebræ, 188.8 (208-179); weight (16 individuals), 113.7 (131.9-98.0); excluding pregnant females, 13 individuals averaged 112.9 grams (131.9-98.0).
Averages for 13 adult males: Total length, 326 millimeters (345-311); tail vertebræ, 187.8 (202-168); weight, 116.8 grams (129-100).
There appears to be no significant difference in the measurements and weights of males and females, with the possible exception of the comparison of adult males and adult nonpregnant females.
OCCURRENCE.
GENERAL DISTRIBUTION.
Dipodomys spectabilis spectabilis is found in southeastern Arizona, in northwestern, central, and southern New Mexico, in extreme western Texas, in northern Sonora, and in northern and central Chihuahua (Fig. 1). A subspecies, D. s. cratodon Merriam, has been described from Chicalote, Aguas Calientes, Mexico, the geographic range of which lies in central Mexico in portions of the States of Zacatecas, San Luis Potosi, and Aguas Calientes.
HABITAT.
In the Tucson region spectabilis is typically a resident of the Lower Sonoran Zone. This is perhaps the principal zone inhabited over its entire range, but the animal is often found in the Upper Sonoran also, and in the Gallina Mountains of New Mexico Hollister found it[8] invading the yellow pine Transition where the soil was dry and sandy and the pine woods of open character. The same observer found it common in grassy and weed-grown parks among the large junipers, pinyons, and scattering yellow pines of the Bear Spring Mountains, N. Mex. Bailey calls attention to the fact that the animal apparently does not inhabit the lower half of the Lower Sonoran Zone, as it extends neither into the Rio Grande Valley of Texas nor the Gila Valley of Arizona. In extreme western Texas it is common at the upper edge of the arid Lower Sonoran Zone, and in this region does not enter the Upper Sonoran to any extent.
In July, 1914, Goldman found this kangaroo rat common on the plain at 4,600 feet altitude, near Bonita, Graham County, Ariz., and noted a few as high as 5,000 feet altitude on the warm southwestern slopes of the Graham Mountains, near Fort Grant. Apparently spectabilis reaches its upper altitude limit in the Burro Mountains, N. Mex., where Bailey has found it sparingly on warm slopes up to 5,700 feet, and at the western base of the Sandia Mountains, east of Albuquerque, N. Mex., where dens occur at approximately 6,000 feet.
About Tucson it is undoubtedly more common in the somewhat higher portions of the Lower Sonoran Zone, above the Covillea association, than elsewhere (Pl. IV, Figs. 1 and 2). A few scattered dens are to be seen in the Covillea belt, but as one rises to altitudes of 3,500 to 4,000 feet, and the Covillea is replaced by the cat's-claws (Acacia sp. and Mimosa sp.) and scattered mesquite (Prosopis), with the Opuntia becoming less abundant, kangaroo rat mounds come more and more in evidence. Here is to be found the principal grass growth supporting the grazing industry, and the presence of a more luxuriant grass flora is probably an important factor in the greater abundance of kangaroo rats, both spectabilis and merriami. In this generally preferred environment the desert hackberry (Celtis pallida) is one of the most conspicuous shrubs; clumps of this species are commonly accompanied by kangaroo rat mounds.
In order to ascertain whether the banner-tailed kangaroo rat has any marked preference for building its mounds under Celtis or some other particular plant, all the observable mounds were counted in a strip about 20 rods wide and approximately 4 miles long, an area of approximately 160 acres, particular note being taken of the kind of shrub under which each mound was located. Of 300 mounds in this area, 96 were under Prosopis, 95 under Acacia, 65 under Celtis, 11 under Lycium, 31 in the open, 1 about a "cholla" cactus (Opuntia spinosior), and 1 about a prickly pear (Opuntia sp.). There is apparently no strongly marked preference for any single species of plant. While both desert hackberry and the cat's-claws afford a better[9] protection than mesquite—since cattle more often seek shade under the latter, and in so doing frequently trample the mounds severely—it appears that the general protection of a tree or shrub of some sort is sought by kangaroo rats, rather than the specific protection of the thickest or thorniest species.
The following records indicate particular habitat preferences of spectabilis as noted at different points in its range:
Occurs on open bare knolls exposed to winds, also on gravelly places at lower edge of foothills (Franklin Mountains, Tex., Gaut); here and there over the barest and hardest of the gravelly mesas (Bailey, Tex., 1905, 147); on open creosote-bush and giant-cactus desert (Tucson, Ariz., Vorhies and Taylor); on firm, gravelly, or even rocky soil on the grassy bajada land along the northwest base of the mountains, either in the open or under Celtis, Prosopis, Lycium, Acacia greggii, or other brush (Santa Rita Mountains, Ariz., Vorhies and Taylor); mounds usually thrown up around a bunch of cactus or mesquite brush (Magdalena, Sonora, Bailey); in heavy soil (Ajo, Ariz., A. B. Howell); loamy soil (Gunsight, Ariz., A. B. Howell); in mesa where not too stony (Magdalena, Sonora, Bailey); grassy plain (Gallego, Chihuahua, Nelson); in open valley and high open plains (Santa Rosa, N. Mex., Bailey); in grassy and weed-grown parks among the larger junipers, pinyons, and scattering yellow pines (Bear Spring Mountains, N. Mex., Hollister); on sand-dune strip (east side of Pecos River, 15 miles northeast of Roswell, N. Mex., Bailey); among Ephedra patches (San Juan Valley, N. Mex., Birdseye); in open sandy soil along dry wash (Rio Alamosa, N. Mex., Goldman); on sides and crests of bare, stony hills (Mesa Jumanes, N. Mex., Gaut); in open, arid part of the valley and stony mesas (Carlsbad and Pecos Valley, N. Mex., Bailey); about the edges of the plains of San Augustine and the foothills of the Datil and Gallina Mountains, and in the Transition Zone yellow-pine forest of the Gallina Mountains (Datil region, N. Mex., Hollister); on hard limy ridges (Monahans, Tex., Cary).
A. Brazier Howell notes that spectabilis occurs in harder soil than does deserti. This observation is confirmed by others, and seems to afford a conspicuous habitat difference between the two, for deserti is typically an animal of the shifting aeolian sands.
Usually, as on the Range Reserve, the rodents are widely distributed over a considerable area. Occasionally, as in the vicinity of Rio Alamosa, N. Mex., as reported by Goldman, they occur only in small colonies.
 Plate IV. Fig. 1.—Range Conditions Favoring Kangaroo Rats.
Plate IV. Fig. 1.—Range Conditions Favoring Kangaroo Rats.View on higher portion of Range Reserve, showing type of country where Dipodomys s. spectabilis is most abundant. Good growth of grama and needle grasses in October, following summer growth and before grazing off by cattle and rodents.
 Plate IV. Fig. 2.—Range Conditions Less Favorable to Kangaroo Rats.
Plate IV. Fig. 2.—Range Conditions Less Favorable to Kangaroo Rats.View on lower portion of Range Reserve, where Dipodomys s. spectabilis is less abundant. Vegetation consists principally of Lycium, mesquite, rabbit brush, and cactus, there being very little grass.
HABITS.
EVIDENCE OF PRESENCE.
Mounds.
One traveling over territory thickly occupied by the banner-tailed kangaroo rat is certain to note the numerous and conspicuous mounds so characteristic of the species, particularly if the region is of the savannah type, grassy rather than brushy. These low, rounded mounds occupy an area of several feet in diameter, and rise to varying[10] heights above the general surface of the surrounding soil, the height depending rather more upon the character of the soil and the location of the mound as to exposure or protection than upon the area occupied by the burrow system which lies within and is the reason for the mound.
A den in sandy soil in the open may be of maximum size in area occupied and yet scarcely present the appearance of a mound in any sense, due probably both to the fact that the sandy soil will not heap up to such a height over a honeycomb of tunnels as will a firmer or rocky soil, and also to its greater exposure to the leveling action of rains and the trampling of animals. These mounds are in themselves large enough to attract some attention, but their conspicuousness is enhanced by the fact that they are more or less completely denuded of vegetation and are the centers of cleared areas often as much as 30 feet in diameter (Pl. V, Fig. 1); and further that from 3 to 12 large dark openings loom up in every mound. The larger openings are of such size as to suggest the presence of a much larger animal than actually inhabits the mound. Add to the above the fact that the traveler by day never sees the mound builder, and we have the chief reasons why curiosity is so often aroused by these habitations.
On the Range Reserve the mounds are usually rendered conspicuous by the absence of small vegetation, but Nelson writes that in the vicinity of Gallego, Chihuahua, they can be readily distinguished at a distance because of a growth of weeds and small bushes over their summits, which overtop the grass. In the vicinity of Albuquerque, N. Mex., Bailey reports (and this was recently confirmed by Vorhies) that the mounds about the holes of spectabilis are often hardly noticeable. Hollister writes that in the yellow-pine forests of the Gallina Mountains the burrows are usually under the trunk of some fallen pine, both sides of it in some cases being taken up with holes, there being some eight or ten entrances along each side, the burrows extending into the ground beneath the log. In the vicinity of Blanco, N. Mex., Birdseye says that occasionally spectabilis makes typical dens but more often lives in old prairie-dog holes (Cynomys), or in holes which look more like those of D. ordii.
Runways and Tracks.
Still other features add to the interest in the dwelling places of spectabilis. Radiating in various directions from some of the openings of the mounds well-used runways are to be seen, some of them fading out in the surrounding vegetation, but others extending 30, 40, or even 50 or more yards to neighboring burrows or mounds (Pl. V, Fig. 2; Pl. VI, Fig. 1). These runways and the entrances to the mounds are well worn, showing that the inhabitants are at home and[11] are at some time of day very active. The worn paths become most conspicuous in the autumnal harvest season, when they stand out in strong contrast to surrounding grass. One usually finds not far distant from the main habitation one or more smaller burrows, each with from one to three typical openings, connected by the trail or runway system with the central den, and these we have called "subsidiary burrows" (Pl. VI, Fig. 2). These will be again referred to in discussing the detailed plan of the entire shelter system.
Examination of the runways and of the denuded area about a mound discloses an abundance of almost indecipherable tracks. The dust or sand is ordinarily much too dry and shifting to record clear footprints, and there are no opportunities to see footprints of this species recorded in good impressionable soil. Very characteristic traces of kangaroo rats may be readily observed in the dust about the mounds, however, and these are long, narrow, sometimes curving, furrows made by the long tails as the animals whisk about their work or play.
 Plate V. Fig. 1.—Clearing About a Mound.
Plate V. Fig. 1.—Clearing About a Mound.A typical clearing about a mound of Dipodomys s. spectabilis, showing the autumnal denudation of the mound and surrounding areas. In this instance about 30 feet in diameter.
 Plate V. Fig. 2.—Mound and Runways.
Plate V. Fig. 2.—Mound and Runways.A small mound of Dipodomys s. spectabilis in early autumn, showing runways radiating from the den. Evidences of activity may be noted in and about the surface of the mound.
 Plate VI. Fig. 1.—Runway of Dipodomys s. spectabilis.
Plate VI. Fig. 1.—Runway of Dipodomys s. spectabilis.Well-traveled path leading from the main den, in the foreground, to a subsidiary burrow (see Fig. 2, below), about 30 feet distant, at apparent end of runway.
 Plate VI. Fig. 2.—Subsidiary Burrow of Dipodomys s. spectabilis.
Plate VI. Fig. 2.—Subsidiary Burrow of Dipodomys s. spectabilis.Located at the end of the 30-foot runway shown in Figure 1, above. This has three openings, two in the foreground and the third a little to the rear and indicated by an arrow.
Signals.
If a scratching or tapping sound be made at the mouth of a burrow, even in the daytime, one is likely to hear a muffled tapping in response, and this may at times be heard while one is engaged in excavating a mound. It has a chirring or fluttering quality, described by Fisher as resembling the noise of a quail flying. Bailey (1905, 148) is of the opinion that it is used as a signal of alarm, call note, or challenge, a view which the present authors believe to be correct. During the winter of 1920-21, however, both Bailey and Vorhies discovered that this sound, or a very similar one, is made by the rapid action of the forefeet in digging. On one occasion in the laboratory the sound was given by one of a pair and was responded to at once by the other, the two being in separate but contiguous cages. This observation, however, could not be repeated. (Vorhies MS.)
One evening, while working in the vicinity of the Burro Mountains, N. Mex., Goldman heard a kangaroo rat near camp making this thumping noise. Taking a lantern, he approached the den, very cautiously, until within 10 feet. The kangaroo rat was just outside the entrance of one of its burrows, and though moving about more or less restlessly at first showed little fear, and kept up the thumping or drumming at intervals. When making the noise the animal was standing with the forefeet on the ground and the tail lying extended. The noise seemed to be made with the hind feet only, and the vibration of the feet could be seen. The tapping was kept up for a second or two at a time, the sounds coming close together and being repeated[12] rhythmically after a very short interval, suggesting the distant galloping of a horse. After continuing in this way for a short time, the animal turned quickly about, with its head in the opposite direction, and began tapping. It appeared to pay little attention to the light, but finally gave a sudden bound and entered one of its holes about 4 feet from the one in front of which it had been standing.
Vorhies has repeatedly noted when watching for the appearance of a kangaroo rat at night that this sound invariably precedes the rodent's first emergence into the open, and often its appearance after an alarm, though when the storage season has begun and the kangaroo rat is carrying loads of grass heads or other material into its den, it regularly comes out without preliminary signaling. Vorhies has also observed it making the sound while on top of the mound, and certainly not digging, but was unable to see how it was made.
Voice.
No data concerning any call notes or sounds other than those described above are at hand, with the following exception: Price (in Allen, 1895, 213), who studied the habits of the animal in the moonlight, at Willcox, Ariz., says that a low chuckle was uttered at intervals; and Vorhies has had one captive female that would repeatedly utter a similar chuckle in a peevish manner when disturbed by day, and one captive male which, when teased into a state of anger and excitement, would squeal much like a cornered house rat. Vorhies has spent many moonlight hours observing kangaroo rats, but without ever hearing a vocal sound uttered by free individuals.
DAILY AND SEASONAL ACTIVITY.
The kangaroo rat is strictly nocturnal. An observer watching patiently by a den in the evening for the animal's first appearance is not rewarded until darkness has fallen completely, and unless the moon is shining the animal can hardly be seen. Were it not for the white tail-brush of spectabilis and its white belly when upright on the hind legs and tail, one could not as a rule see the animal at all when it makes its first evening appearance. With the first streak of dawn activity usually ceases completely and much more abruptly than it began with the coming of darkness, but on a recent occasion Vorhies observed that a kangaroo rat which did not appear until near morning remained above ground until quite light, but not fully daylight. On removal of the plug from the mouth of a kangaroo rat burrow, one may sometimes see a fresh mass of earth and refuse shoved into the opening from within. As often as not, however, even this unwelcome attention does not elicit any response by day, the great majority of the burrow openings of this species, as observed by the authors, remaining permanently open.
[13]The ordinary activities of the kangaroo rat in southern Arizona can scarcely be said to show any true seasonal variation. The animals are active all the year in this region, there being neither hibernation nor estivation, both perhaps being rendered unnecessary by the storage habit, to be discussed in full later (pp. 15-16), and by the mildness of the winter climate. On any particular night that the weather is rainy, or the ground too wet and cold, activity is confined to the interior of the burrow system, and for this reason one has no opportunity to see a perfect imprint of the foot in freshly wet soil or in snow. On two or three of the comparatively rare occasions on which there was a light fall of snow on the Range Reserve a search was made for tracks in the snow. At these times, however, as on rainy nights, the only signs of activity were the pushing or throwing out of fresh earth and food refuse from within the burrow. This is so common a sight as to be complete evidence that the animals are active within their dens during stormy weather but do not venture outside. Trapping has again and again proved to be useless on rainy nights, unless the rain is scant and a part of the night favorable, in which case occasional individuals are taken. These statements apply to the Range Reserve particularly; the facts may be quite different where the animals experience more winter, as at Albuquerque, N. Mex., although in November, 1921, Vorhies noted no indications of lessened activity in that region.
PUGNACITY AND SOCIABILITY.
So far as their reactions toward man are concerned, kangaroo rats are gentle and make confiding and interesting pets; this is especially the case with merriami. This characteristic is the more surprising in view of the fact that they will fight each other so readily and so viciously, and yet probably it is explained in part by their method of fighting. They do not appear to use their teeth toward each other, but fight by leaping in the air and striking with the powerful hind feet, reminding one most forcibly of a pair of game cocks, facing each other and guarding in the same manner. Sometimes they carry on a sparring match with their fore feet. Biting, if done at all, is only a secondary means of combat. When taken in hand, even for the first time, they will use their teeth only in the event that they are wounded. The jaws are not powerful, and though the animals may lay hold of a bare finger, with the apparent intention of biting, usually they do not succeed in drawing blood. As Bailey says (1905, 148), they are gentle and timid, and, like rabbits, depend upon flight and their burrows for protection.
The well-traveled trails elsewhere described (p. 10) indicate a degree of sociability difficult to explain in connection with their pugnacity[14] toward each other. While three or four individuals may sometimes be trapped at a single mound, more than two are seldom so caught, and most often only one in one night. Trapping on successive nights at one mound often yields the larger number, yet in some cases the number is explained by the fact that two or three nearly mature young are taken, and the capture of several individuals at a single mound can not be taken to indicate that all are from the one den. Our investigations tend strongly to the conclusion that only one adult occupies a mound, except during the period when the young are in the parental (or maternal) den. In the gassing and excavating of 25 or more mounds we have never found more than one animal in a den, except in one instance, and then the two present were obviously young animals.
SENSE DEVELOPMENTS.
Without making special investigations through a study of behavior or other special methods, one can speak in only general terms regarding what appear to be the special sense developments of kangaroo rats. The eyes are large, as is very often the case in nocturnal animals, and when brought out into the bright light of day the rats perhaps do not see well. Yet, if an animal leaves a den which is in process of excavation, and follows one runway, even in bright sunlight, it makes excellent speed to the next opening, often a distance of several yards. Whether this is accomplished chiefly by the aid of sight or in large measure by a maze-following ability, such as experiments have shown some rodents to have, can not be stated without precise experimentation. Marked ability to follow a maze would not be at all surprising in view of the labyrinthine character of the underground passages which make up the normal habitation.
When watching beside a mound by moonlight one is impressed with the fact that the rats possess either a very keen sense of hearing or of sight, probably both. The very slightest movement or noise on the part of the observer results, with a timid individual, in an instantaneous leap for safety, a disappearance into the burrow so sudden as to be almost startling. All attempts to obtain flashlight photographs at the mounds were failures, the animal either having gotten completely out of the field before the light flashed following the pull of the trigger, or leaving merely an indistinguishable blur on the plate as it went, and this in spite of carefully hiding the trigger chain behind a screen. A slight noise accompanying the trigger action gave the alarm in one case, and in another the length of time of the flash was sufficient for the get-away. The marvelous quickness of the animal clearly indicates a remarkably short reaction time.[15] Occasionally a bold individual is found, as in the case of one which came out repeatedly, even after being flashed twice in the same night.
Certain peculiar physical characteristics suggest a relationship to sense reactions. On these, however, the authors are not prepared to do more than offer suggestions for future work. The extremely large mastoids found in kangaroo rats suggest a connection in some way with special developments of the sense of hearing or of balance. It may be noted that an intermediate condition between the kangaroo rats and the majority of rodents in respect to this character is to be found in the pocket mice (Perognathus), which belong to the same family. Herein lies a field for some interesting experimentation and discovery.
The small, pointed nose might suggest a not overkeen sense of smell, and there appears no reason to believe that this sense is particularly well developed. However, the turbinals are very complex. The vibrissæ are long and sensitive, and may indicate a special development of the sense of touch as an adaptation to nocturnal habits and to life in an underground labyrinth. The long, well-haired tail doubtless serves as an important tactile organ as well as a balance.
MOVEMENTS AND ATTITUDES.
Movements and attitudes are characteristic. As a kangaroo rat emerges from the burrow a reason for the relatively large size of the opening is seen in the fact that, kangaroolike, the animal maintains a partially upright position. Its ordinary mode of progression is hopping along on the large hind legs, or, when in the open and going at speed, leaping. When moving slowly about over the mound, as if searching for food, it uses the fore legs in a kind of creeping movement. It appears to be creeping when pocketing grain strewn about, but close observation shows that the fore feet are then used for sweeping material into the pockets, reminding one somewhat of a vacuum cleaner. When it assumes a partially upright position the fore limbs are usually drawn up so closely that they can be seen only by looking upward from a somewhat lower level than that occupied by the animal. The slower movements of searching or playing about the mound are occasionally interrupted by a sudden leap directly upward to a height of 1-1/2 to 2 feet, often with no apparent reason other than play. This is, however, a fighting or guarding movement, though indulged in for play. The play instinct seems to be well developed, and in evidence on any moonlight night when actual harvesting operations are not going on.
STORING HABITS.
Probably no instinct is of greater importance to the kangaroo rat than that of storing food supplies. When a crop of desirable seeds[16] is maturing the animal's activities appear to be concentrated on this work. During September, 1919, when a good crop of grass seed was ripening following the summer rains, a kangaroo rat under observation made repeated round trips to the harvest field of grass heads. Each outward trip occupied from 1 to 1-1/2 minutes, while the unloading trip into the burrow took only 15 to 20 seconds.
One individual in a laboratory cage, which had not yet been given a nest box, busied itself in broad daylight in carrying its grain supply into the darkest corner of the cage. When a nest box is supplied the individual will retreat into its dark shelter, and will only come forth after darkness has fallen unless forcibly ejected, but will store the food supplied.
In another case an animal escaped while being handled, and sought refuge behind a built-in laboratory table, where it could not be recovered without tearing out the table. For four days and nights it had the run of the laboratory. On the first night of its freedom it found and entered a burlap bag of grass seed that had been taken from a mound. A trail of seed and chaff next morning showed that it had been busily engaged in making its new quarters comfortable with bedding and food. After four nights of freedom it was captured alive in a trap, and later it was found that it had moved from the corner behind the table to the space beneath a near-by drawer, where it had stored about 2 quarts of the grass seed and a handful of the oatmeal used for trap bait.
BREEDING HABITS.
Observations on breeding habits have consisted mainly in taking records from the females trapped at all seasons of the year throughout the course of the investigation, and from examinations made during poisoning operations, and yet from this source the number of pregnant females taken or of young discovered is disappointingly small. The records indicate a breeding period of considerable length, extending from January to August, inclusive. It is possible that the length of the period may be increased by a second litter from the earliest breeding females in summer, but the large percentage of nonpregnant or nonbreeding animals which occurs throughout the season would indicate a wide variation in the time of breeding of different individuals.
Trapping in February and March for the purpose of securing greater numbers of female specimens, begun with the idea that these months were most likely to be the breeding months, has invariably yielded an unsatisfactory number of nonbreeding specimens and males. Unfortunately, the numbers of females secured in some months were not sufficient to be significant if worked out in percentages[17] of breeding and nonbreeding individuals, and this, coupled with the fact that the importance of recording carefully all nonbreeders was not at first recognized, makes it impossible to tabulate such information reliably. The total of females taken in April, for example, is only 3, of which 1 was breeding; while in June, during the course of poisoning operations, 45 females were examined, of which 21 were breeding.
Five breeding females were taken in January, all during the last three days of the month. One of these was a suckling female, the young of which were secured alive and were probably at least a week old when taken. This must have been exceptionally early for young, since of a number of adult kangaroo rats taken during the first week of January none have been found to be breeding. Two records from Vernon Bailey are as follows: May 19-June 8, 1903, young specimen in nest (Santa Rosa, N. Mex.); June 12, 1889, one female, two embryos (Oracle, Ariz.).
The considerable proportion (which we believe to be more than 50 per cent) of nonbreeding females taken during all those months in which breeding has been found to occur may also indicate an extended period of breeding, with a small percentage breeding at any one time. This period also furnishes ample time for the rearing of two litters a year by some females, but we have no evidence as to the occurrence of two litters. Young of the year, practically grown, are taken during and after the month of April.
The mammae are arranged in three pairs, pectoral, 1/1; inguinal, 2/2.
Kangaroo rats are among those rodents in which the vagina becomes plugged with a rather solid material, translucent, and of the consistency of a stiff gelatine, after copulation. This must occur very soon after coitus, since in those individuals taken in this condition no definite evidence of the beginning of development of embryos could be detected by examination.
The length of the gestation period of spectabilis is unknown. The young are born naked, a fact inferred by failure to find any fetus showing noticeable hair development, and from the conditions observed in such young as have been seen. A suckling female was taken by Vorhies, January 31, 1920, and her den immediately excavated in the hope of securing the young. Two juveniles were found in a special nest chamber (see p. 30). These were estimated to be perhaps two weeks old. A serious effort was made to raise the little animals by feeding milk with a pipette and keeping them warm with a hot water bottle, but they survived only 10 days, without the eyes having opened. The uneven temperature as well as the character of the food was probably responsible for their deaths. On February 3 they were measured and weighed, with the following results:
| Weight (in grams). | Measurements (in millimetres). | |||
| Total length. | Tail vertebrae. | Hind foot. | ||
| No. 1 | 13.3 | 90 | 38 | 24 |
| No. 2 | 12.6 | 93 | 38 | 24 |
At this stage the young were partially clothed with a coat of fine velvety fur, more especially on the bodies, the tails being still nearly naked. The body color was dark plumbeous, just the color of the dark underfur of the adult, or a shade darker, while the characteristic white markings of the adult stood out sharply as pinkish-white areas against the dark background (see Pl. IX, Fig. 2, at p. 32). The proportions were much as in the adult, except that the tails were relatively much shorter and the feet relatively longer.
Only one other record of young is at hand, that by Bailey, who secured the young after capture of a suckling female at Santa Rosa, N. Mex. In this case the litter contained only one. This was squeaking when found, but was not large enough to crawl away. Its eyes and ears were closed, and its soft, naked skin was distinctly marked with the pattern of the adult, the colors being as given for the other two. This juvenile lived only a week. Young less than half grown were not trapped or noted in our poisoning operations outside the dens.
Kangaroo rats, if spectabilis be representative, reproduce at a slow rate as compared with many other small rodents. We have records of 67 females with embryos or scars showing the number produced, and of the two litters of young described above. Of the 69 females thus recorded, 15, or 21.7 per cent, had but one offspring each; 52, or 75.3 per cent, but two each; while only 2 individuals, or 2.9 per cent, had three. Three young is the maximum litter recorded. This, taken in connection with the protracted breeding season and lack of sure evidence of the production of two broods a year, gives a surprisingly low rate of reproduction, indicating relative freedom from inimical factors.
Our breeding records for merriami are fewer than for spectabilis, but are very similar in every way so far as they go, both as to the time of year and number of young.
FOOD AND STORAGE.
Dipodomys s. spectabilis does not hibernate, so must prepare for unfavorable seasons by extensive storage of food materials. There are two seasons of the year, in southeastern Arizona at least, when storage of food takes place, namely, in spring, during April or May, and in fall, from September to November, the latter being the more important. For the periods between, the animal must rely largely[19] on stored materials. Not infrequently a season of severe drought precludes the possibility of any storage. The summer and fall of 1918 was such a season on the Range Reserve (Pl. II, Fig. 2). If food stores are inadequate at such a time the kangaroo rats must perish in considerable numbers. Fisher found many deserted mounds in the vicinity of Dos Cabezos, Ariz., in June, 1894, which may be accounted for in this way. In 1921 Vorhies found all mounds within 4 or 5 miles of Albuquerque, N. Mex., deserted by spectabilis, resulting probably from overgrazing by sheep and goats during a succession of dry years. In the arid Southwest natural selection probably favors the animals with the largest food stores, and it is not surprising that the storing habit has been developed to a remarkable degree.
Some stored material is likely to be found at any time of year in any mound examined, the largest quantity usually in fall and winter, the smallest in July or August (Table 1, dens 1, 2, 14, and 24). Amounts found by different observers vary from a few ounces to several quarts or pecks, and stored materials taken from 22 mounds on the Range Reserve vary in weight from 5 to 4,127 grams (more than 9 pounds). This is exceeded by one lot from New Mexico, which totaled 5,750 grams (12.67 pounds). It is fairly evident that in seasons of scanty forage for stock the appropriation of such quantities of grass seeds and crowns and other grazing materials by numerous kangaroo rats may appreciably reduce the carrying capacity of the range. Studies of cheek-pouch contents and food stores taken from dens show that the natural food of spectabilis consists principally of various seeds and fruits, particularly the seeds of certain grasses. The study of burrow contents has been especially illuminating and valuable.
All of the stored material from 22 dens on the Range Reserve and from 2 near Albuquerque, N. Mex., has been saved and analyzed as to species as carefully as the conditions of storage would permit. Within the mound the food stored is usually more or less segregated by plant species, though the stores of material of any one kind may be found in several places through the mound, and often the material is mixed. In the latter case the quantities of the various species can only be estimated, but in the former the species may be kept separate by the use of several bags for collecting the seeds, and a fairly accurate laboratory weighing can be made later. Very frequently, the explanation of this separation of species lies in the different seasons of ripening, but sometimes where two species are ripe at the same time near the mound, one is worked upon for a time to the exclusion of the other. The one kind is often packed in tightly against the other, but with a very abrupt change in the character of the material.
[20]A number of the more interesting and representative results of the weighing and analyses of burrow contents are presented herewith in tabular form. The data for each den, or lot, shows in grams the quantity of stored material removed and the best estimate it was possible to make of the percentages or weights of the various species. When the weight was less than 5 grams, the mere trace of the species frequently is indicated in the following tables by the abbreviation "Tr."
Table 1.—Analyses of plants stored by Dipodomys spectabilis spectabilis Merriam, obtained from examination of representative dens (all except Den 24 from U. S. Range Reserve, near the Santa Rita Mountains, Ariz.).
Den 1.
February 7, 1918. Burrow typical, located on bank of wash in partially denuded grass-land, Bouteloua rothrockii and weed type; soil sandy; burrow photographed in section (Pl. VII, Fig. 1).
| Species stored. | Grams. |
| Bouteloua rothrockii | 2,205 |
| Bouteloua aristidoides (B. eriopoda and B. rothrockii, Tr.) | 1,445 |
| Plantago ignota | 442 |
| Eriogonum polycladon | 35 |
|
|
|
| Total | 4,127 |
Four species of plants represented in burrow contents (Pl. VII, Fig. 2). Maximum quantity for single burrow in series of 22 from Range Reserve.
Den 2.
March 9, 1918. Surroundings overgrazed and partially restored by complete protection. Red soil, with much coarse rough gravel and stone.
| Species stored. | Grams. | |
| Bouteloua rothrockii (nearly pure) | 1,460 | |
| Bouteloua rothrockii (mixed with Aristida spp.) | 945 | |
| Boerhaavia wrightii | 660 | |
| Bouteloua rothrockii | } | 525 |
| Bouteloua aristidoides | ||
| Aristida divaricata | ||
| Aristida bromoides | ||
| Kallstroemia laetevirens | Tr. | |
| Heterotheca subaxillaris | Tr. | |
| Plantago ignota | 15 | |
| Fleshy fungi | 10 | |
|
|
||
| Total | 3,615 |
Eight species of plants represented by seeds. One species of fleshy fungus in addition.
Den 4.
September 20, 1918. In Calliandra type. Stony or gravelly soil, red, nearly denuded of grass.
| Species stored. | Grams. | ||
| Prosopis velutina | 190 | ||
| Mollugo verticillata (pure) | 90 | ||
| Anisolotus trispermus (mixed, but mostly of this genus) | 50 | ||
| Solanum elaeagnifolium (12 fruits) | 2 | ||
| Per cent. | |||
| Mollugo verticillata (inseparable) | 50 | } | 400 |
| Bouteloua rothrockii | 1 | ||
| Bouteloua aristidoides | 10 | ||
| Lepidium lasiocarpum | Tr. | ||
| Polygala puberula | Tr. | ||
| Ayenia microphylla | 2 | ||
| Portulaca suffrutescens | 1 | ||
| Aplopappus gracilis | Tr. | ||
| Alternanthera repens | 1 | ||
| Tridens pulchella | 1 | ||
| Plantago ignota | 33 | ||
| Panicum hallii | Tr. | ||
| Fleshy fungi (puffballs) | 2 | ||
|
|
|||
| Total | 734 |
Fifteen species represented in addition to the fleshy fungi. No perceptible grass growth from the summer rains here, therefore dependent on a wide variety of scattering plants.
Den 6.
October 17, 1918. Mixed type, partially denuded, no growth from summer rains. Sandy soil.
| Species stored. | Grams. |
| Bouteloua rothrockii (crowns) (heads 1 to 2 per cent) | 1,435 |
| Bouteloua rothrockii (heads and crowns, about 50 per cent of each) | 325 |
| Bouteloua rothrockii (with small percentage of crowns) | 315 |
| Boerhaavia wrightii (with a few grass crowns) | 150 |
| Prosopis velutina | 90 |
| Solanum elaeagnifolium (3 fruits) | Tr. |
|
|
|
| Total | 2,315 |
Four species represented. Count of 100 grams of stored Bouteloua crowns gives 1,700, or 17 crowns per gram. At this rate there were at least 27,000 crowns stored in this burrow. If a density of 250 plants to the square yard be assumed (a high estimate) these crowns represent the total B. rothrockii on 104 square yards of range surface. Further examination of the vicinity of this den showed that the surrounding area was not completely cleared, but was devoid of B. rothrockii, while still having B. eriopoda with crowns undisturbed.
Den 11.
April 9, 1919. In partially denuded land where good spring growth of Eschscholtzia was in bloom at time of excavation. Stomach of spectabilis killed[22] in this burrow contained a mass of fresh but finely comminuted green material, probably poppy leaves, strongly colored with yellow from blossoms. No summer growth here in 1918.
| Species stored. | Grams. | |
| Bouteloua rothrockii (crowns) (miscellaneous chaff, etc.) | 107 | |
| Eschscholtzia mexicana (buds and flowers) | } | 10 |
| Anisolotus trispermus (leaves and pods) | ||
| Gaertneria tenuifolia (leaves) | ||
| Lupinus sparsiflorus (flowers) | ||
| Solanum elaeagnifolium (2 fruits) | Tr. | |
|
|
||
| Total | 117 |
Six species represented, some only by leaves or flowers and not by seeds. Such storage is never in large quantity. The fresh storage material was weighed after becoming air dry. This illustrates a late spring condition, storage running low.
Den 14.
August 8, 1919. Excellent summer growth all over range. This burrow in mixed growth, grasses and weeds.
| Species stored. | Grams. |
| Miscellaneous portions of green plants of mixed species, no seeds | 5 |
Representing minimum for any one of the 22 burrows studied. Active storage does not begin until September.
Den 16.
October 17, 1919. In good grass, but mound overrun by a large Apodanthera vine.
| Species stored. | Per cent. | Grams. | |
| Aristida divaricata | 90 to 95 | } | 58 |
| Chamaecrista leptadenia | 10 to 5 | ||
| Bouteloua rothrockii | Tr. | ||
| Prosopis velutina | 200 | ||
| Apodanthera undulata | 55 | ||
|
|
|||
| Total | 313 |
Five species represented. Two species, Apodanthera, and Chamaecrista leptadenia, new to storage records. Several whole fruits of Apodanthera, about 2 inches in diameter, stored in addition to seeds alone; seeds of this form not previously noted in burrows, but very abundant in this one, indicating importance of the factor of accessibility in storage.
Den 19.
October 31, November 1, 1919. In good grass. Entire burrow system mapped (Fig. 2, p. 29).
| Species stored. | Per cent. | Grams. | |
| Aristida spp. (probably mostly divaricata) | 98 | } | 1,813 |
| Eriogonum sp | Tr. | ||
| Bouteloua rothrockii | 1 | ||
| Bouteloua aristidoides | 1 | ||
| Panicum sp | Tr. | ||
| Prosopis velutina | 1,213 | ||
|
|
|||
| Total | 3,026 |
[23]Five species represented, in addition to those of Aristida. Largest storage of Prosopis found. Mound was near a good-sized mesquite tree. No storage in subsidiary burrows.
Den 21.
January 31, 1920. Male trapped here night of January 29, and suckling female trapped at same place and same opening of mound, night of January 30. Burrow excavated to secure young, which were found in special nest chamber.
| Species stored. | Grams. |
| Aristida spp. (intimate mixture of undetermined species) | 1,115 |
| Eschscholtzia mexicana (from spring of 1919) | 48 |
| Opuntia (prickly pear, seeds only, no fruits) | 10 |
|
|
|
| Total | 1,173 |
Three species represented. Prickly pear hitherto found as fruits only.
Den 22.
January 1, 1921. Rather good grass growth here in summer of 1920. Burrow typical, sandy soil. Two skulls of former residents unearthed.
| Species stored. | Grams. |
| Aplopappus gracilis (some B. rothrockii) | 1,030 |
| Astragalus nuttallianus | 630 |
| Bouteloua rothrockii (some A. gracilis) | 530 |
| Sida diffusa | 30 |
| Solanum elaeagnifolium (282 fruits) | 53 |
| Loeflingia pusilla | Tr. |
| Bouteloua aristidoides | Tr. |
| Plantago ignota | Tr. |
| Lupinus sparsiflorus | Tr. |
| Old storage (mostly Bouteloua aristidoides with traces of B. rothrockii and Aristida divaricata) | 60 |
|
|
|
| Total | 2,333 |
Eleven species represented. First instance of quantity storage of Aplopappus gracilis. First occurrence of Loeflingia pusilla and Astragalus nuttallianus.
Den 24.
November 8, 1921. On mesa northeast of Albuquerque, N. Mex., near base of Sandia Mountains. Fair grass growth here during preceding summer.
| Species stored. | Grams. |
| Sporobolus cryptandrus strictus | 5,455 |
| Salsola pestifer | 295 |
|
|
|
| Total | 5,750 |
Two species represented. The heads of Sporobolus cryptandrus strictus are retained to a great extent within the leaf sheaths. This necessitates the cutting of the stems into suitable lengths for carrying, and the stored material appears to be merely cut sections of the stems. Close examination, however, discloses the heads within, and shows that as in other instances seed storage is the end sought. These pieces are packed beautifully parallel like so many matches,[24] and vary from a minimum length of 20 to a maximum of 37 millimeters, averaging about 30. Count of 2 grams of the above Sporobolus material shows that there are 125 separate cut sections per gram, or a total of approximately 680,000 pieces in this one lot of storage, indicating a remarkable activity on the part of the individual rat (Pl. VIII, Fig. 1).
 Plate VII. Fig. 1.—Den Excavated on Range Reserve.
Plate VII. Fig. 1.—Den Excavated on Range Reserve.Vertical section through Den No. 1, of Table 1 (p. 20), showing the complex system of burrows, some of them plugged with closely packed storage (outlined in white), the depth of the den, and the widened chambers centrally located.
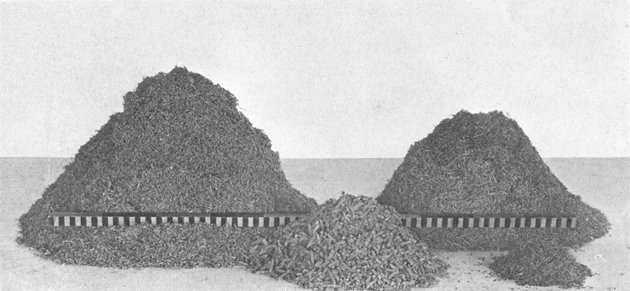 Plate VII. Fig. 2.—Content of Excavated Den.
Plate VII. Fig. 2.—Content of Excavated Den.Storage content of Den No. 1 (Fig. 1, above), showing the separate species of plants listed in Table 1. The rod is 1 meter long. The large pile on the left is composed of seed-laden heads of crowfoot grama (Bouteloua rothrockii), the large pile on the right consists of heads of six-weeks grama (Bouteloua aristidoides), the pile of heads in the center is desert plantain (Plantago ignota), and the smallest heap is composed of buckwheat-bush seeds (Eriogonum polycladon).
The number of lots of storage (24) studied in detail, extending as it does over a period of three years with seasons of varying growth conditions, is not sufficient to permit the construction of a curve showing increase and decrease in quantity of stored material with growing seasons and intervals between; but the results indicate a very decided increase during the autumn storing season, and continuing large well into the winter, since some outside material can still be obtained until midwinter. From about February to April a decrease may be noted, followed, if the spring growth of annuals be good, by a slight increase; and we can very nearly predict the general character of the increases and decreases by the precipitation and consequent growth conditions.
Table 2.—Quantity of storage per den correlated with time of year and growth conditions of preceding season (chiefly from United States Range Reserve near the Santa Rita Mountains, Ariz.).
| Den No. | Date. | Quantity. | Preceding season. |
| 1918. | Grams. | ||
| 1 | Feb. 7 | 4,127 | Good. |
| 2 | Mar. 9 | 3,615 | Do. |
| 3 | July 25 | 401 | Poor. |
| 4 | Sept. 20 | 734 | Do. |
| 5 | Sept. 21 | 2,520 | Do. |
| 6 | Oct. 17 | 2,315 | Do. |
| 7 | Dec. 20 | 1,247 | Do. |
| 1919. | |||
| 8 | Feb. 7 | 1,600 | Do. |
| 9 | Mar. 13 | 370 | Do. |
| 10 | Apr. 7 | 180 | Do.[3] |
| 11 | Apr. 9 | 117 | Good.[3] |
| 12 | May 7 | 298 | Do.[3] |
| 13 | May 11 | 1,590 | Do. |
| 14 | Aug. 8 | 5 | Good. |
| 15 | Sept. 4 | 151 | Do. |
| 16 | Oct. 17 | 313 | Do. |
| 17 | Oct. 18 | 583 | Do. |
| 18 | Oct. 25 | 3,410 | Do. |
| 19 | Nov. 1 | 3,026 | Do. |
| 20 | Dec. 13 | 2,816 | Do. |
| 1920. | |||
| 21 | Jan. 31 | 1,173 | Do. |
| 1921. | |||
| 22 | Jan. 1 | 2,333 | Fair. |
| 23[4] | Nov. 7 | 1,685 | Good. |
| 24[4] | Nov. 8 | 5,750 | Do. |
In presenting Table 2, showing quantity of storage per burrow correlated with the time of year and the character of the preceding growing season, the fact may be emphasized that the growing seasons in southern Arizona are two in number—early spring and midsummer. The spring season is the less important, the plants consisting chiefly of a variety of small annuals, while the important range grasses make their chief growth and head out almost exclusively in the July-August rainy season. It may be noted also that the actual[25] increases in storage appear somewhat after the growth period proper, since storing does not get well under way until the seed crop is mature. The banner-tailed kangaroo rat shows a marked adaptability to different foods available in the neighborhood of its burrows. It must, perforce, adapt itself and its storage program to the food that it can get, and this varies enormously with the climatic conditions of successive seasons. The large numbers present in suitable localities clearly indicate that the animal is successful in meeting the changing and sometimes extremely adverse conditions of its environment.
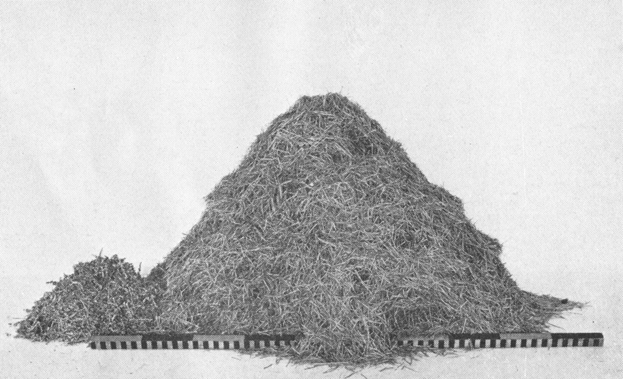 Plate VIII. Fig. 1.—Content of Den Excavated in New Mexico.
Plate VIII. Fig. 1.—Content of Den Excavated in New Mexico.Storage content of Den No. 24, of Table 1, from Sandia Mountains, N. Mex. This is the largest lot of storage taken in the course of the investigations. The larger pile consists wholly of a valuable grass, Sporobolus cryptandrus strictus: the smaller of Russian thistle (Salsola pestifer.)
 Plate VIII. Fig. 2.—Growth Following Elimination of Kangaroo Rats.
Plate VIII. Fig. 2.—Growth Following Elimination of Kangaroo Rats.The same mound as shown in Plate III, Figure 1, after three years of protection, the rodents having been killed out. Nearly as good grass recovery following poisoning operations occurred in the single excellent season of 1921.
At times, more especially in the seasons of active growth, some of the green and succulent portions of plants are eaten. This was very noticeable in the spring of 1919, when a most luxuriant growth of Mexican poppy (Eschscholtzia mexicana) occurred. Stomachs at this time were filled with the yellow and green mixture undoubtedly produced by the grinding up of the buds and flowers of this plant. Small caches of about a tablespoonful of these buds were also found in the burrows at this time. Occasionally in spring one may find a few green leaves of various plants, Gaertneria very commonly, tucked away in small pockets along the underground tunnels, indicating that such materials are used to some extent. As has been shown in detail, however (Table 1), the chief storage, and undoubtedly the chief food, consists of air-dry seeds.
The character of the storage, the absence of rain for months at a time in some years, and the consequent failure of green succulents show that without doubt spectabilis possesses remarkable power, as to its water requirements, of existing largely if not wholly upon the water derived from air-dry starchy foods, i.e., metabolic water serves it in lieu of drink (Nelson, 1918, 400), this being formed in considerable quantities by oxidation of carbohydrates and fats (Babcock, 1912, 159, 170). During the long dry periods characteristic of southern Arizona, no evidence that the animal seeks a supply of succulent food, as cactus, is found; and if it may go for two, three, or six months without water or succulent food, it is reasonable to suppose that it may do so indefinitely. In the laboratory spectabilis ordinarily does not drink, but rather shows a dislike for getting its nose wet. During the periods of drought the attacks upon the cactuses by other rodents of the same region, as Lepus, Sylvilagus, Neotoma, and Ammospermophilus, become increasingly evident. The list of plant species thus far found represented in the storage materials of spectabilis on the Range Reserve is shown in Table 3.
[26] Table 3.—List of all plant species found in 22 dens of Dipodomys spectabilis on the United States Range Reserve, near the Santa Rita Mountains, Ariz., with approximate total weights.
| Grasses. | |
| Grams. | |
| Aristida bromoides (six-weeks needlegrass) | 536 |
| Aristida divaricata (Humboldt needlegrass) | 9,412 |
| Aristida scabra (rough needlegrass) | 344 |
| Bouteloua aristidoides (six-weeks grama) | 3,093 |
| Bouteloua radicosa (grama) | 1,269 |
| Bouteloua eriopoda (black grama) | Tr. |
| Bouteloua rothrockii (seeds, 8,495; crowns, 3,517 grams) (crowfoot grama) | 12,012 |
| Festuca octoflora (fescue grass) | 70 |
| Panicum arizonicum (Arizona panic-grass) | 11 |
| Panicum hallii (Hall panic-grass) | Tr. |
| Pappaphorum wrightii | Tr. |
| Tridens pulchella | Tr. |
| Valota saccharata | Tr. |
| Other Plants. | |
| Alternanthera repens | Tr. |
| Anisolotus trispermus (bird's-foot trefoil) | 186 |
| Aplopappus gracilis | 1,030 |
| Apodanthera undulata (melon loco) | 55 |
| Astragalus nuttallianus (milk vetch) | 630 |
| Ayenia microphylla | Tr. |
| Boerhaavia wrightii | 885 |
| Chamaecrista leptadenia (partridge pea) | 5 |
| Echinocactus wislizeni (visnaga) | 5 |
| Eriogonum polycladon | 35 |
| Eschscholtzia mexicana (Mexican poppy) | 250 |
| Gaertneria tenuifolia (franseria) | Tr. |
| Collomia gracilis (false gilia) | Tr. |
| Heterotheca subaxillaris | Tr. |
| Kallstroemia laetevirens | Tr. |
| Lupinus sparsiflorus (lupine) | Tr. |
| Martynia altheaefolia (small devil's-horns) | 12 |
| Mollugo verticillata (carpetweed) | 324 |
| Oenothera primiverus (evening primrose) | 15 |
| Opuntia discata (prickly pear) | 15 |
| Loeflingia pusilla | Tr. |
| Lepidium lasiocarpum (peppergrass) | Tr. |
| Plantago ignota (plantain) | 818 |
| Polygala puberula (milkwort) | Tr. |
| Portulaca suffrutescens (purslane) | Tr. |
| Prosopis velutina (mesquite) | 1,570 |
| Sida diffusa (spreading sida) | 30 |
| Solanum elaeagnifolium (742 fruits) (trompillo, prickly solanum) | 156 |
| Puffballs and fleshy fungi (undetermined) | 12 |
Total species, exclusive of fungi, 41.
[27]It will be seen from Table 3 that while a large number of species of plants are represented in the totals from so many dens, a majority of them are of very minor importance, and that the seeds of grasses are the principal storage and probably therefore the principal food material. Six of the most important species of grasses (disregarding species furnishing less than 5 grams) comprise 85.6 per cent of the total weight of storage from 22 dens. Crowfoot grama (Bouteloua rothrockii) stands first in quantity in the total, forming 39.4 per cent of all stored material, 46 per cent of the six important grasses, and 45 per cent of all grasses. The largest amount of storage of any one species of grass in any one den on the Range Reserve also is of this species, 2,205 grams[5] (Table 1, den 1, p. 20, and Pl. VII, Fig. 2). This is exceeded by a dropseed grass, Sporobolus cryptandrus strictus, which amounted to 5,455 grams in a lot from Albuquerque, N. Mex. (Table 1, den 24, and Pl. VIII, Fig. 1).
Of the species other than grasses found stored in these dens, mesquite beans (Prosopis velutina) are most important both by weight and number of dens containing them. The total for the 22 Range Reserve dens is 1,570 grams, or 35.9 per cent of the seeds other than grasses, but only 5.1 per cent of the total storage. In bulk mesquite beans do not loom up large, as they are probably the heaviest material stored. Sections of pods which must have been dragged into the burrows are found, some of them certainly being much too long for carriage in the pouches. The species of plant other than grass found in the largest quantity in any one den, however, was Aplopappus gracilis, not recorded in quantity from any den until the excavation of the twenty-second, and then found in a very large bulk of soft, fluffy material, with most of the seeds separated from the heads, and weighing 1,030 grams (Table 1, den 22).
Any of the food materials above listed are likely to be found in the cheek pouches, while in addition such extraneous matter as stones and feces have also been found. All species of plants stored are accessible in the immediate vicinity of the mound, and when any particular plant is found seeding in abundance in the vicinity of the den it is likely to be represented in the storage. Usually the animals can be readily trapped with almost any kind of grain bait, as oats, rolled oats, rolled barley, and wheat; and nut meats also are attractive, though we have no record of the storing of any true nut in the dens, such not being available in the range of the animal on the Range Reserve.
[28]The following plants not represented in the list stored by the kangaroo rat on the Range Reserve have been found in the cheek pouches or mounds of spectabilis in other localities:
Amaranthus palmeri, Sesuvium portulacastrum, and Atriplex wrightii (alluvial soil of Santa Cruz Valley, Continental, Ariz., Bailey).
Cut leaves and stems of a small sagebrush (Franklin Mountains, Tex., Gaut).
Gutierrezia heads (San Juan Valley, N. Mex., Birdseye).
Verbesina enceliodes, Portulaca oleracea, Bouteloua gracilis, and Munroa squarrosa (Rio Alamosa, N. Mex., Goldman).
Tops of buds of Artemisia filifolia (Mesa Jumanes, N. Mex., Gaut).
Tumbleweed (Amaranthus graecizans), Russian thistle (Salsola pestifer), Munroa squarrosa, and Sporobolus cryptandrus strictus (Sandia Mountains, Albuquerque, N. Mex., Vorhies).
BURROW SYSTEMS, OR DENS.
The burrow system, or den, in which spectabilis stores its caches of food materials, has its nest, and remains throughout the hours of daylight is a complicated labyrinth of tunnels. Ejection of refuse and soil from this retreat builds up the mound frequently referred to. These mounds are, as Bailey says, characteristic of the species, and are as unmistakable as muskrat houses or beaver dams, and as carefully planned and built for as definite a purpose—home and shelter. They are, furthermore, the most notable of all kangaroo rat dwelling places (Nelson, 1918, 400). They range in height from 6 inches to approximately 4 feet and from 5 to 15 feet in diameter.
The mound is built up not only through the cleaning out of chaff and other food refuse, but through extension and modification of the tunnels; old tunnels, entrances, and caches of musty food material are from time to time closed up and others excavated, repair and rebuilding being especially necessary after the collapse of portions of the den as a result of heavy rains or trampling by cattle. Ejected material is most commonly simply thrown out fan-wise from the openings, without much apparent effort to add to the height of the mound.
There are usually from 6 to 12 entrance holes in each mound opening into the subterranean burrow system, each hole from 4 to 5-1/2 inches in diameter. These holes are nearly all situated a little above the surface of the surrounding soil, and as Price has suggested (in Allen, 1895, 213), this is doubtless a wise provision against flooding, as torrential rains sometimes occur in the kangaroo rat country.
Both Bailey and Nelson state that as a rule several of the holes are closed with sand or miscellaneous earth and old storage material during the daytime, but our observations on the Range Reserve are that such closing is only occasional. Many occupied dens have not[29] a single opening closed. Further, night observations disclose that the inhabitant of the mound will appear from some one of the two or three most-used openings when night falls, and not necessarily from one which has been closed by day. Recently an opening closed one day was observed in use during the night, but was left open all the next day.
In attempting to determine whether there exist similarities of plan or system in the dens, it was considered advisable to map them with some degree of accuracy. This we were enabled to do by laying off a square about a given mound, 2-1/2 or 3 meters each way, and subdividing it into a series of small squares of half a meter on each side by drawing cross-lines on the surface of the ground over the top of the mound. One person then did the digging and exploring of the tunnels, as to direction and depth, while the other noted the results on coordinate paper (Figs. 2 and 3); the proper excavation and mapping of one of these workings occupied from four to eight hours for the two.
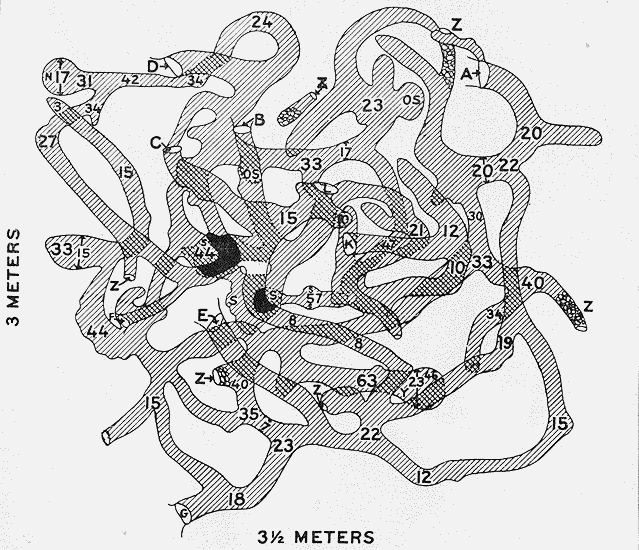 Fig. 2.—Diagram of a typical den of Dipodomys spectabilis spectabilis. Double shading indicates where one portion of tunnel lies above another and solid black a three-story arrangement; A, B, C, etc., active openings to surface; figures without arrows, depths in centimeters to tunnel roofs; figures with arrows, tunnel widths in centimeters; N. nest chamber; S, storage; OS, old storage; Y, probably an old nest chamber; Z, old, unused, or partially plugged openings.
Fig. 2.—Diagram of a typical den of Dipodomys spectabilis spectabilis. Double shading indicates where one portion of tunnel lies above another and solid black a three-story arrangement; A, B, C, etc., active openings to surface; figures without arrows, depths in centimeters to tunnel roofs; figures with arrows, tunnel widths in centimeters; N. nest chamber; S, storage; OS, old storage; Y, probably an old nest chamber; Z, old, unused, or partially plugged openings.[30]While there is greater complexity in the larger, and probably older, mounds than in the smaller, all are extremely complicated and can only be described as labyrinthine in character. The tunnels wind about through the mound, rising and falling in vertical depth, intercommunicating frequently, but with occasional cul-de-sacs, and in places expanding into chambers, of which there may be three or four large ones. The stored materials are found in some, but not necessarily all, of these chambers, and may also occupy considerable lengths of ordinary tunnel, especially when the quantity present is large. Small evaginations of the tunnels frequently contain lesser caches, and it is in such pockets that bits of fresh material are placed during a growing season, or that grain supplied the previous night for bait is usually found.
The main masses of storage are most often found centrally located at depths of from 15 to 57 centimeters, although at times one may find a cache near the periphery of the system and as near the surface as 2 or 3 centimeters. In the latter case the materials are subject to wetting from rains, and consequent spoilage.
The major portion of the whole tunnel system is within about 50 centimeters of the surface of the mound, but usually some one branch tunnel goes to somewhat greater depth, and this is likely to be the one containing the nest; this is also likely to extend toward or beyond the periphery of the main system, and always ends blindly. Such a one, from which two young were taken on January 31, 1920, was at a depth of about 65 centimeters, and about 1-1/2 meters beyond the periphery of the mound itself.
The individual tunnels average about 8 centimeters in height, and about 11 centimeters in width, though the variation, especially in width, is considerable. The expansions mentioned as being the chief places of storage are from 15 to 25 centimeters in diameter, and may or may not involve a considerable increase in height. They are frequently located at junction points of two or more branches of the tunnel system.
The nest cavity is a chamber of approximately spherical shape and from 17 to 23 centimeters in diameter. Chambers of this character were observed and noted as "old storage" in a number of cases. They were sometimes cut off from the rest of the habitation, and at first were supposed to contain abandoned musty storage. As experience in excavating and interpreting results has been gained we have concluded that these chambers in fact represent abandoned nests.
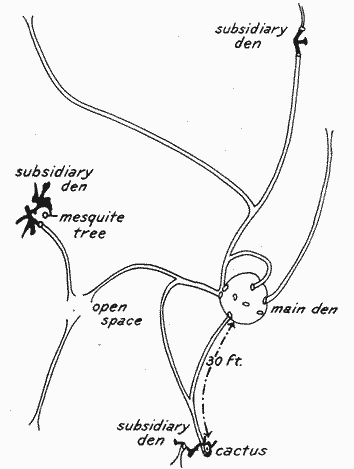 Fig. 3.—Diagram of the system of surface runways and subsidiary dens of Dipodomys spectabilis spectabilis. The underground tunnels of the main den were too complicated to illustrate on this scale, being very similar to those of Figure 2. The underground tunnels of the subsidiaries are shown in solid black. Some runways fade out in the grass in a manner that can not be indicated in a line drawing.
Fig. 3.—Diagram of the system of surface runways and subsidiary dens of Dipodomys spectabilis spectabilis. The underground tunnels of the main den were too complicated to illustrate on this scale, being very similar to those of Figure 2. The underground tunnels of the subsidiaries are shown in solid black. Some runways fade out in the grass in a manner that can not be indicated in a line drawing.Bailey gives the dimensions of nest chambers observed in New Mexico as about 6 by 8 inches to 8 by 10 inches. The nest is composed of finer, softer, and more chaffy material than the regular[31] storage. The chaff refuse from the food probably contributes largely to it, though some leaves of grasses not stored for food may also be found, and a nest, especially the one in use, may be distinguished, if excavating is carefully done, by the distinct cavity about the size[32] of a fist in its interior (Pl. IX, Fig. 1). One may sometimes find this cavity distinctly warm from the recent presence of the inhabitant.
The walls or partitions between the chambers and tunnels are in places surprisingly thin, and it is no wonder that one is almost certain to break through in stepping on a mound, since the whole is a honeycomblike structure of from two to four stories in vertical plan, as shown by the transect of a mound in Plate VII, Figure 1. As Bailey writes, these partition walls are a mixture of earth and old food and nest material discarded years ago, resembling the adobe walls of the Mexican houses built of chopped earth and straw. This is the result of the continual ejection of refuse and earth as before mentioned, combined with the caving action of rains and disturbances from larger animals.
Apparently there are no special pockets for deposit of feces in Dipodomys burrows; such matter may be found throughout the den, and is more or less mixed with the food refuse which carpets practically the entire tunnel system. The nest and food stores are, however, clean and neat, the droppings being dry and, though present on the floor of a storage chamber, not actually mingled with the food. Evidently the animal does not clean up the floor litter before storing food material.
The entire system for any one den seems to consist not only of the burrows within the mound itself, as described, but of those small outlying ones which we have referred to as subsidiary burrows. These are two to four in number, and are connected with the main mound by the runways already mentioned. They often seem to be way stations on the runways connecting main mounds, and there is seldom any mound of earth whatever in connection with them. One entire den system, the home mound and three subsidiaries, was mapped after being excavated (Fig. 3), all having been carefully gassed with carbon bisulphide. The subsidiaries were simple and contained no storage. Two of them were shallow, while in the third a depth of 48 centimeters was reached. They appear to be merely places of refuge, though the well-worn trails connecting them with the main mound indicate regular use. These runways are conspicuous on the Range Reserve, and are apparently characteristic of mounds throughout the range of the animal. Dwellers in different mounds must have rather extensive social contacts, notwithstanding the enmity of individuals toward each other in captivity. The main mound, in this instance very complicated, was in one place three stories high, and we have found as many as four utilized stories; but as a rule there are two or three only.
Since collapses are rather frequent during rainy seasons, aside from the trampling previously referred to, the kangaroo rats, where[33] abundant, as on the Range Reserve, may well be a factor in increasing soil porosity and fertility; for in the course of time they probably have succeeded in plowing and cultivating the whole surface layer of the soil. They may thus be a factor in ecologic succession, tending to improve the character of the soil and adapt it to another stage.
Doubtless their own workings afford the only shelter the animals know. In the course of our digging in one mound, the occupant, an adult male, did not forsake the den until the excavation was three-fourths completed; and even then it did not leave by a burrow leading away from our operations, but came toward us, escaped the active efforts of four individuals bent on its capture, and ran speedily along a used runway toward another burrow several meters distant. A sack had been stuffed in the mouth of this, however, and, baffled, the rat then returned to the original burrow and was captured. Observations on other rats thus driven from the home mound indicate that they are very familiar with the runways of the vicinity of the mound and the various subsidiary burrows, and it is a question whether they need to see clearly to follow these runs. Apparently they never attempt to escape by forsaking their well-traveled runways. Tests of the maze-running ability of these animals by animal-behavior experts would be of extraordinary interest, in view of the character of the homes which they always inhabit and the network of runs on the outside.
 Plate IX. Fig. 1.—Kangaroo Rat Nest and Young.
Plate IX. Fig. 1.—Kangaroo Rat Nest and Young.Nest and the two young, the ordinary number in the litter, of Dipodomys s. spectabilis, taken from den on January 31, 1920.
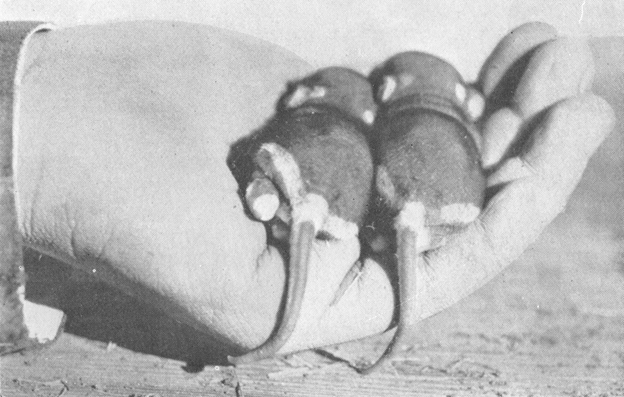 Plate IX. Fig. 2.—Young of the Kangaroo Rat.
Plate IX. Fig. 2.—Young of the Kangaroo Rat.The same young as shown in Figure 1, above. They were probably about two weeks old, the pelage being short but with the white markings of the adult; the tails are relatively short and with scarcely any hair.
COMMENSALS AND ENEMIES.
COMMENSALS.
It is doubtful whether any animals live in a truly commensal relationship with spectabilis, but of not unfriendly associates there are a great number. It is the experience of Bailey, corroborated by observations of Vorhies on living animals, that these kangaroo rats are active in defending their caches of food, and will even fight individuals of the same species savagely and to the death. One moonlight night a strange individual was liberated on a mound. It deliberately entered one of the openings, but after about two minutes' time made an exceedingly rapid exit, running rapidly out of sight as if pursued, though the owner of the home did not appear outside of the burrow. There can be little doubt that the stranger was precipitately ejected by the owner. We suspect, though this is a point difficult to prove satisfactorily, that merriami does not always store food supplies for itself, but visits the burrows of spectabilis regularly to pilfer the seed stored therein. The observed facts thus far recorded which suggest this are that in no merriami burrow examined has a store of food been found, and also that in[34] trapping for spectabilis on its own characteristic mounds one catches a large percentage of merriami.
On two separate occasions Vorhies has observed the smaller species running over the mounds of the larger, actually carrying away the grain which had been placed to entice the larger when it might appear. (In these cases the larger species did not put in an appearance until near morning.) Furthermore, the dens of merriami are often connected by distinct runways with those of spectabilis, indicating much traveling or visiting. That this is probably not friendly visiting is suggested by the certainty with which an individual of the larger species will strike and kill one of the smaller when they are placed together in the same inclosure. The word "thief" expresses this suspected relationship better than would the term "parasite."
It is not to be expected that such obvious shelter retreats as the mounds of spectabilis should fail to attract the attention of other animals. We have found a small gecko (Coleonyx variegatus), scorpions of two or three undetermined species, and certain insects (of the Order Orthoptera) to be very common inhabitants of the dens. With the exception of the parasitic insects the most common are wingless locustids (Ceuthophilus spp.) and the peculiar wingless females of a species of cockroach (Arenivaga erratica). These two are seldom absent when a burrow is excavated, the female cockroaches being abundant, although the winged males have never been taken in the burrows.
Cary's observations at Monahans, Tex., and those of others at numerous localities, combined with our own, show that at various times the dens furnish protection and shelter for various species of cottontail rabbits (Sylvilagus), ground squirrels (Citellus and Ammospermophilus), wood rats (Neotoma), grasshopper mice (Onychomys), rattlesnakes (Crotalus), and most of the common lizards. Of these the ground squirrels Citellus tereticaudus and Ammospermophilus harrisii are most often noted on the Range Reserve using the dens as a retreat, the Ammospermophilus seldom being observed to enter any other kind of burrow. It should be added that the total observations include dens which have been deserted by their rightful owners.
NATURAL CHECKS.
The enemies of the kangaroo rat are not determined in detail, or as to relative importance, but the badger (Taxidea taxus berlandieri) and the kit fox, or swift (Vulpes macrotis neomexicana), may well be foremost. Dens which have been deeply excavated by badgers are frequently seen, and sometimes two or three badger tunnels penetrate one burrow system. Dens thus despoiled are[35] probably soon reoccupied even if the original owner is captured, and in the course of a few months the reworking of the abode obliterates the signs of destruction.
Droppings of the kit fox show an abundance of bones of small mammals of kangaroo rat size, among them those of spectabilis.
Bobcats (Lynx baileyi) and coyotes (Canis mearnsi) probably are a prejudicial factor. Skunks may sometimes be able to surprise the kangaroo rats, but probably not often. The western horned owl (Bubo virginianus pallescens), the barn owl (Tyto alba pratincola), and perhaps others may well be among the most feared enemies, but no special investigation of owl pellets on the reserve has been possible. In 592 barn-owl pellets from California were found remains of 230 kangaroo rats, only one other rodent being represented by a larger number (McAtee, 1921, 258).
Much more information on enemies is needed. The relatively low rate of reproduction (see p. 18) indicates comparative freedom from inimical factors.
PARASITES.
Dipodomys s. spectabilis is regularly infested with a species of flea, Ctenophthalmus sp. Seldom or never is a specimen taken in reasonably fresh condition without some of these parasites present on its body, though of course they desert the body of the host after it becomes cold, and hence dead specimens left too long may be free from them. The den conditions are ideal for the breeding of this parasite, because of the great quantities of fine, dusty, organic refuse littering the tunnels and furnishing food and refuge for the larvæ. As demonstrated to us by F. C. Bishopp, of the Bureau of Entomology, a handful of this refuse taken from the floor of a burrow within arm's length of the entrance is almost certain to contain these larvæ.
Less regularly present, perhaps because of its different life history, is a small tick, Trombicula sp. At times this parasite is very common, being present on nearly every individual rat, and at other times specimens are difficult to find; it appears to be more commonly present in summer and fall than at other seasons, and is found attached chiefly to the ears.
No internal parasites have been detected. The nocturnal and fossorial habits of the animal seem to give complete protection against a form of parasite which is very common among some other rodents of the Range Reserve, notably Lepus and Sylvilagus. Nearly all rabbits are infested with "warbles," the larvæ of a species of bot-fly, Cuterebra (family Oestridae). Other small mammals also are occasionally parasitized by the Cuterebra, but in the handling and examination of perhaps 200 or more individuals of spectabilis and merriami, we have yet to find a single case of infestation by an oestrid fly.
[36]ABUNDANCE.
One's first impression of a well-occupied spectabilis area is that a large family must inhabit each den, but, as previously mentioned, we have gradually been compelled to shift from this conception to the idea of but a single animal to a mound, except when the young are present. Therefore a census of the adult kangaroo rat population can readily be made, simply by counting the mounds. Such a census affords at least a conservative estimate of the number of adult individuals occupying a given area.
The first estimates of abundance on the Range Reserve were from actual counts of dens on areas measured off for experimental fencing, and gave the figure of about two mounds to the acre. From time to time rough estimates were made on different portions of the pastures, and these checked well with the above. Later still, a careful count showed 300 mounds on approximately 160 acres (see p. 8), or 1.87 mounds per acre. Nine areas of 2 acres each, representing different environmental conditions, were later selected in different portions of the Range Reserve, and the dens accurately counted. The number of dens per 2 acres varied from none to a maximum infestation of 12, neither extreme occurring over large areas. The total number of dens was found to be 43 on the 18 acres, or an average of 2.38 dens per acre.
From all these estimates it may fairly be concluded that two mounds, or two animals, per acre is a conservative estimate for the infestation of the entire Range Reserve, with the possible exception of small areas at its upper edges, where the altitude limit of spectabilis is passed. It is, however, impossible to estimate the area of the State infested with kangaroo rats, for some large stretches of fine grassland show no kangaroo rats whatever, while others have more than are present on the reserve; and we have no estimates of the extent of either type.
ECONOMIC CONSIDERATIONS.
In May, 1894, Fisher found a ranchman at Willcox, Ariz., who complained more bitterly of the depredations of spectabilis than of those of any other mammal.
On the United States Range Reserve the food material appropriated by the kangaroo rat during good years is inappreciable. There is such an excess of forage grass produced that all the rodents together make very little difference. But with the periodic recurrence of lean years, when drought conditions are such that little or no grass grows, the effects of rodent damage not only become apparent, but may be a critical factor determining whether a given number of domestic animals can be grazed on the area (Pl. VIII, Fig. 2).
[37]With two kangaroo rats to the acre (1,280 per square mile), there would be 64,000 animals on the 50 square miles of the Range Reserve. If each rat stores 4 pounds of grass seeds and crowns and other edible forage during the season (and in severe seasons we find that more crowns are stored than under ordinary conditions), a total of 256,000 pounds, or 128 tons, of edible forage are rendered unavailable to stock. In dry years it is probable that this amount of forage would be of critical importance. Allowing 50 pounds of food a day for each steer, the forage destroyed would be sufficient to provide for the needs of one steer for 5,120 days, or for the needs of 14 steers for one year. On a stock ranch the size of the Range Reserve this might mean the difference between success and failure.
It seems not unlikely, therefore, that during seasons of drought the banner-tailed kangaroo rat, where it is abundant on the grazing ranges of the Southwest, may be a factor of critical importance in relation to forage production and carrying capacity. It must be remembered, moreover, that the stored material consists largely of seeds, so that this loss is of greater importance than would be the case were it ordinary forage. Some of the range grasses of this region found in greatest quantity in the stored material depend in large part, under certain conditions, upon seed reproduction. Rehabilitation of a depleted range after severe drought and consequent close grazing and trampling is retarded by the heavy toll of seed taken by the kangaroo rats.
CONTROL.
Kangaroo rats may be easily eradicated by the use of the poisoned grain used for prairie-dog control by the Biological Survey and the University of Arizona Extension Service. This can be obtained by application to the State representative of the Biological Survey or to the local county agricultural agent, or may be mixed as follows:
Formula for poisoned bait.—Dissolve 1 ounce of strychnine sulphate in 1-1/2 pints of boiling water. Add 1 heaping tablespoonful of gloss starch, previously mixed with a little cold water, and boil until a clear paste is formed. Add 1 ounce of baking soda and stir to a creamy mass. Add 1/2 ounce of glycerine and 1/4 pint of corn sirup and stir thoroughly. Pour over 16 quarts of rolled barley and mix well until every grain is evenly coated. Allow to dry before using.
In bushel quantities use as above directed, 2 ounces of strychnine, 2 ounces of soda, 1 ounce of glycerin, 1-1/4 ounces of starch, 1-1/2 quarts of boiling water, and 5/8 pint of corn sirup.
Scatter poison, when the natural food of the kangaroo rat is scarce, on clean hard places near the holes, 1 quart to 50 holes.
If powdered strychnine alkaloid is used, prepare the hot starch paste first. Then sift strychnine and baking soda, previously thoroughly mixed, into the hot starch paste and stir to a creamy mass. Proceed as in the above directions with sirup, glycerin, etc.
[38]Use this poison within five days after mixing or retain in air-tight containers.
Caution.—All poison containers and all utensils used in the preparation of poison should be kept plainly labeled and out of reach of children, irresponsible persons, and live stock.
A spoonful of the poisoned grain scattered about the used entrances of a mound is sufficient, and prebaiting is not necessary, as with prairie dogs.
A word of caution should perhaps be offered in connection with control measures. As man has come to occupy a greater portion of the earth's surface, and as he has become more and more the master of his environment, he has inevitably disturbed the relationships of the birds and mammals about him, has upset the balance of nature. If he kills the carnivorous species because of their depredations on game and live stock he must be prepared to cope with the increased hordes of rodents which feed on vegetation and on which the carnivorous animals act as a check. If he destroys the rodents, he may remove the checks on certain noxious plants or insects. One control measure often necessitates the adoption of another.
This is not to argue against control measures, for if our harmful species were not controlled, agriculture in many sections would be impossible. Control measures, however, should be scientifically founded and applied. The indiscriminate slaughter of supposedly harmful species of birds and mammals in the guise of benefiting agriculture may do far more harm than good. Many of the species which do some harm do far more good. The exact status of each suspected species should be carefully determined through an adequate scientific investigation. If the species is condemned, sound control measures should be thoroughly applied.
In grazing districts or in areas devoted to intensive agriculture the death sentence should probably be passed on the banner-tailed kangaroo rat. It should be recalled, however, that this is the largest and one of the handsomest of all its family, and that it is one of the most characteristic and interesting of all the desert fauna; where extensive grazing or agricultural operations are not undertaken, therefore, we feel that the kangaroo rat should be let alone, unless its presence threatens infestation of valuable agricultural or grazing lands.
SUMMARY.
(1) Kangaroo rats may be separated with ease from all other mammals; the long tail and short and weak fore feet separate them from the pocket gophers; the white hip-stripe distinguishes them from the pocket mice. The decidedly larger size and the white-tipped tail separate Dipodomys spectabilis spectabilis and D. deserti from D.[39] merriami and D. ordii. The darker color and vividly contrasted black-and-white tail of spectabilis distinguish it from deserti.
(2) Dipodomys s. spectabilis occurs in the open arid country of portions of the Lower and Upper Sonoran Zones of Arizona, New Mexico, Texas, Sonora, and Chihuahua. It lives in harder soil than does deserti, and builds more conspicuous mounds.
(3) There is no evidence of intergradation or hybridization between spectabilis and deserti.
(4) Dipodomys s. spectabilis is nocturnal; it is gentle, and does not offer to bite when taken in the hand; is silent for the most part; active; somewhat sociable with its fellows, but fights in defense of its food stores; progresses chiefly by leaping; signals by a drumming or tapping on the ground with its hind feet.
(5) The breeding season of spectabilis begins in January and continues into August. Whether more than one litter is raised in a single season is unknown. The number of young in each litter varies from 1 to 3, averaging 2.
(6) Dipodomys s. spectabilis does not hibernate, but provides food stores, mostly seeds, for use during seasons when food would be otherwise unavailable. Storage in each den varies in quantity from 5 grams (about 1/6 ounce) to 5,750 grams (12.67 pounds). Materials stored include several important forage plants; for example, various species of Bouteloua and Aristida, with B. rothrockii (crowfoot grama) the most important. Accessibility and abundance of different plants have much to do with the kinds of storage found.
(7) The dens of spectabilis are the most notable of all kangaroo rat dwelling places. They range from 6 inches to 4 feet in vertical height, and from 5 to 15 feet in diameter. Here the kangaroo rat has its home, shelter, and food-storage chambers. Within the den is found a tortuous network of burrows, with many storage and some nest chambers, the whole arranged so as to be two to four stories high.
(8) Dipodomys s. spectabilis is not of great economic significance, except locally, in ordinary seasons. During periods of extreme drought it may be of critical importance on grazing areas from the standpoint of the carrying capacity of the range.
(9) Kangaroo rats are easy to poison by following the same formula as that used by the Biological Survey for destroying prairie dogs.
(10) In many places unsuited to extensive grazing or agriculture spectabilis does no appreciable damage. It is one of the most interesting of all the rodents peculiar to our Southwestern deserts, and should not be molested except where it is destructive.
BIBLIOGRAPHY.
Allen, J. A.
1895. On a collection of mammals from Arizona and Mexico, made by Mr. W. W. Price, with field notes by the collector. Bull. Amer. Mus. Nat. Hist., vol. 7, art. 6, pp. 193-258. 17 figs. in text.
Babcock, S. M.
1912. Metabolic water: Its production and rôle in vital phenomena. Research Bull. No. 22, Univ. Wisconsin Agr. Exp. Station, pp. 159 and 170, March.
Bailey, V.
1905. Biological survey of Texas. North Amer. Fauna No. 25, Biol. Surv., U. S. Dept. Agr., pp. 222, 16 pls., 24 figs. in text.
Clements, F. E.
1905. Research methods in ecology. Lincoln, Univ. Pub. Co., pp. xvii, 334, 85 figs. in text.
Griffiths, D.
1910. A protected stock range in Arizona. Bull. No. 177, Bur. Plant Ind., U. S. Dept. Agr., pp. 28, 6 pls., 1 fig. in text.
Grinnell, Joseph.
1921. Revised list of the species in the genus Dipodomys. Journal of Mammalogy, vol. 2, No. 2, pp. 94-97, May 2.
McAtee, W. L.
1921. Farm help from the birds. In Yearbook of the U. S. Dept. Agr. for 1920, pp. 253-270; unnumbered figs. in text.
Merriam, C. H.
1890. Description of three new kangaroo rats, with remarks on the identity of Dipodomys ordii of Woodhouse. In North Amer. Fauna No. 4, Div. Ornith. and Mamm. (Biol. Surv.), U. S. Dept. Agr., 41-49.
Nelson, E. W.
1918. Smaller mammals of North America. Nat. Geog. Mag., vol. 33, No. 5, pp. 371-493; numerous unnumbered figs. and colored pls. in text.
ADDITIONAL COPIES
OF THIS PUBLICATION MAY BE PROCURED FROM
THE SUPERINTENDENT OF DOCUMENTS
GOVERNMENT PRINTING OFFICE
WASHINGTON, D. C.
AT
15 CENTS PER COPY
FOOTNOTES:
[1] References in parentheses are to the Bibliography, p. 40 (the last figure being to the page of the publication). References to authorities where no citation of literature is appended relate for the most part to manuscript notes in the files of the Biological Survey or the University of Arizona Agricultural Experiment Station.
[2] In addition to assistance rendered by officials of the Biological Survey and the University of Arizona, which is hereby acknowledged, the authors are indebted to the following persons for helpful suggestions and assistance: G. S. Miller and J. W. Gidley, of the U. S. National Museum; Dr. Frederic E. Clements and Gorm Loftfield, of the Carnegie Institution; Morgan Hebard, of the Academy of Natural Sciences of Philadelphia; James T. Jardine and R. L. Hensel, both formerly connected with the U. S. Forest Service; and R. R. Hill, of the Forest Service. They are also indebted to William Nicholson, of Continental, Ariz., for many courtesies extended in connection with work on the Reserve.
[3] Changing from poor summer season of 1918 to excellent spring growth of 1919.
[4] From near the Sandia Mountains, N. Mex.; others from United States Range Reserve, near the Santa Rita Mountains, Ariz.
[5] This amount of dry grama grass seed (heads) amounts to approximately a bushel.
