University of Kansas Publications
Museum of Natural History
Volume 15, No. 5, pp. 205-249, pls. 7-10, 6 figs.
 October 4, 1963
October 4, 1963 
Amphibians and Reptiles of the Rainforests of Southern El Petén, Guatemala
BY
WILLIAM E. DUELLMAN
University of Kansas
Lawrence
1963
University of Kansas Publications, Museum of Natural History
Editors: E. Raymond Hall, Chairman, Henry S. Fitch,
Theodore H. Eaton, Jr.
Vol. 15, No. 5, pp. 205-249, pls. 7-10, 6 figs.
Published October 4, 1963
University of Kansas
Lawrence, Kansas
PRINTED BY
JEAN M. NEIBARGER, STATE PRINTER
TOPEKA, KANSAS
1963
29-5935
Amphibians and Reptiles of the Rainforests of Southern El Petén, Guatemala
BY
WILLIAM E. DUELLMAN
CONTENTS
| PAGE | ||
| Introduction | 207 | |
| Acknowledgments | 208 | |
| Description of Area | 208 | |
| Physiography | 209 | |
| Climate | 209 | |
| Vegetation | 209 | |
| Gazetteer | 210 | |
| The Herpetofauna of the Rainforest | 211 | |
| Composition of the Fauna | 212 | |
| Ecology of the Herpetofauna | 212 | |
| Relationships of the Fauna | 217 | |
| Accounts of Species | 218 | |
| Hypothetical List of Species | 246 | |
| Summary | 247 | |
| Literature Cited | 247 | |
INTRODUCTION
Early in 1960 an unusual opportunity arose to carry on biological field work in the midst of virgin rainforest in southern El Petén, Guatemala. At that time the Ohio Oil Company of Guatemala had an air strip and camp at Chinajá, from which place the company was constructing a road northward through the forest. In mid-February, 1960, J. Knox Jones, Jr. and I flew into El Petén to collect and study mammals, reptiles, and amphibians. While enjoying the comforts of the fine field camp at Chinajá, we worked in the surrounding forest and availed ourselves of the opportunity to be on hand when the road crews were cutting the tall trees in the forest, thereby bringing to the ground many interesting specimens of the arboreal fauna. We stayed at Chinajá until late March, with the exception of a week spent at Toocog, another camp of the Ohio Oil Company located 15 kilometers southeast of La Libertad and on the edge of the savanna. Thus, at Toocog we were able[208] to work both in the forest and on the savanna. In the summer of 1960, John Wellman accompanied me to El Petén for two weeks in June and July. Most of our time was spent at Chinajá, but a few days were spent at Toocog and other localities in south-central El Petén.
Many areas in Guatemala have been studied intensively by L. C. Stuart, who has published on the herpetofauna of the forested area of northeastern El Petén (1958), the savannas of central El Petén (1935), and the humid mountainous region to the south of El Petén in Alta Verapaz (1948 and 1950). The area studied by me and my companions is covered with rainforest and lies to the north of the highlands of Alta Verapaz and to the south of the savannas of central El Petén. A few specimens of amphibians and reptiles were obtained in this area in 1935 by C. L. Hubbs and Henry van der Schalie; this collection, reported on by Stuart (1937), contained only one species, Cochranella fleischmanni, not present in our collection of 77 species and 617 specimens.
Acknowledgments
I am grateful to L. C. Stuart of the University of Michigan, who made the initial arrangements for our work in El Petén, aided me in the identification of certain specimens, and helped in the preparation of this report. J. Knox Jones, Jr. and John Wellman were able field companions, who added greatly to the number of specimens in the collection. In Guatemala, Clark M. Shimeall and Harold Hoopman of the Ohio Oil Company of Guatemala made available to us the facilities of the company's camps at Chinajá and Toocog. Alberto Alcain and Luis Escaler welcomed us at Chinajá and gave us every possible assistance. Juan Monteras and Antonio Aldaña made our stay at Toocog enjoyable and profitable. During our visits to southern El Petén, Julio Bolón C. worked for us as a collector, and between March and June he collected and saved many valuable specimens; his knowledge of the forest and its inhabitants was a great asset to our work. Jorge A. Ibarra, Director of the Museo Nacional de Historia Natural in Guatemala assisted us in obtaining necessary permits and extended other kindnesses. To all of these people I am indebted for the essential parts that they played in the completion of this study.
Field work in the winter of 1960 was made possible by funds from the American Heart Association for the purposes of collecting mammalian hearts. My field work in the summer of 1960 was supported by a grant from the Graduate Research Fund of the University of Kansas.
DESCRIPTION OF THE AREA
A vast lowland region stretches northward for approximately 700 kilometers from the highlands of Guatemala to the Gulf of Mexico. The northern two-thirds of this low plain is bordered on three sides by seas and forms the Yucatán Peninsula. The lowlands[209] at the base of the Yucatán Peninsula make up the Departamento El Petén of Guatemala. The area with which this report is concerned consists of the south-central part of El Petén.
Physiography
Immediately south of Chinajá is a range of hills, the Serrania de Chinajá, having an almost due east-west axis and a crest of about 600 meters above sea level. South of the Serrania de Chinajá are succeedingly higher ridges building up to the Meseta de Cobán and Sierra de Pocolha and eventually to the main Guatemalan highlands. The northern face of the Serrania de Chinajá is a fault scarp dropping abruptly from about 650 meters at the crest to about 140 meters at the base. From the base of the Serrania de Chinajá northward to the Río de la Pasión at Sayaxché the terrain is gently rolling and has a total relief of about 50 meters. North of the Río de la Pasión is a low dome reaching an elevation of 170 meters at La Libertad; see Stuart (1935:12) for further discussion of the physiography of central El Petén. The rocks in southern El Petén are predominately Miocene marine limestones; there are occasional pockets of Pliocene deposits. There is little evidence of subterranean solution at Chinajá, but northward in central El Petén karsting is common. The upper few inches of soil is humus rich in organic matter; below this is clay.
Climate
The climate of El Petén is tropical with equable temperatures throughout the year. Temperatures at Chinajá varied between a night-time low of 65° F. and a daytime high of 91° F. during the time of our visits. In the Köppen system of classification the climate at Chinajá and Toocog is Af. Rain falls throughout the year, but there is a noticeable dry season. To anyone who has traveled from south to north in El Petén and the Yucatán Peninsula, it is obvious from the changes in vegetation that there is a decrease in rainfall from south to north. There is a noticeable difference between Chinajá and Toocog. Although rainfall data are not available for Chinajá and Toocog, there are records for nearby stations (Sapper, 1932). At Paso Caballos on the Río San Pedro about 40 kilometers northwest of Toocog the average annual rainfall amounts to 1620 mm.; the driest month is March (21 mm.), and the wettest months are June (269 mm.) and September (265 mm.). At Cubilquitz, Alta Verapaz, about 35 kilometers south-southwest of Chinajá and at an elevation of 300 meters, the average annual rainfall is 4006 mm.; the driest month is March (128 mm.), and the wettest months are July (488 mm.) and October (634 mm.).
During the 18 days in February and March, 1960, that we kept records on the weather at Chinajá moderate to heavy showers occurred on seven days. During our stay there in June and July rain fell every day, as it did in Toocog. However, during the week spent at Toocog in March no rain fell.
Vegetation
The vegetation of northern and central El Petén has been studied by Lundell (1937), who made only passing remarks concerning the plants of the southern part of El Petén. No floristic studies have been made there. The[210] following remarks are necessarily brief and are intended only to give the reader a general picture of the forest. I have included names of a few of the commoner trees that I recognized.
Chinajá is located in a vast expanse of unbroken rainforest. In this forest there is a noticeable stratification of the vegetation. Three strata are apparent; in the uppermost layer the tops of the trees are from 40 to 50 meters above the ground. The spreading crowns of the trees and the interlacing vines form a nearly continuous canopy over the lower layers. Among the common trees in the upper stratum are Calophyllum brasiliense, Castilla elastica, Cedrela mexicana, Ceiba pentandra, Didalium guianense, Ficus sp., Sideroxylon lundelli, Swietenia macrophylla, and Vitex sp. (Pl. 1, fig. 1). The middle layer of trees have crowns about 25 meters above the ground; these trees in some places where the upper canopy is missing form the tallest trees in the forest. This is especially true on steep hillsides. Common trees in the middle layer include Achras zapote, Bombax ellipticum, Cecropia mexicana, Orbignya cohune, and Sabal sp. The lowermost layer reaches a height of about 10 meters; in many places in the forest this layer is absent. Common trees in the lower stratum include Crysophila argentea, Cymbopetalum penduliflorum, Casearia sp., and Hasseltia dioica.
The ground cover is sparce; apparently only a few small herbs and ferns live on the heavily shaded forest floor. Important herpetological habitats include the leaf litter, rotting stumps, and rotting tree trunks on the forest floor and the buttresses of many of the gigantic trees, especially Ceiba pentandra (Pl. 2). Epiphytes, especially various kinds of bromeliads, are common. Most frequently these are in the trees in the upper and middle strata.
At Toocog there is sharp break between savanna and forest (Pl. 7, fig. 2). The forest is noticeably drier and more open than at Chinajá (Pl. 9). The crowns of the trees are lower, and there is no nearly continuous canopy between 40 and 50 meters above the ground. Although Swietenia macrophylla and other large trees occur, they are less common than at Chinajá. Especially common at Toocog are Achras zapote, Brosimum alicastrum, and various species of Ficus.
GAZETTEER
The localities from which specimens were obtained are cited below and shown on the accompanying map (Fig. 1).
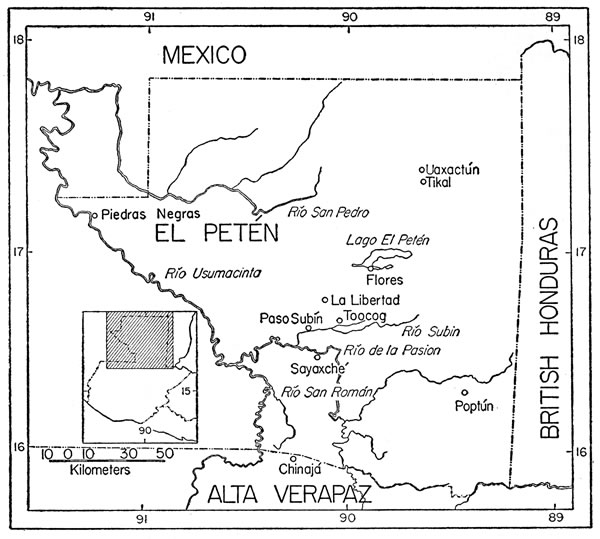 Fig. 1. Map of El Petén, Guatemala, showing localities mentioned in text.
Fig. 1. Map of El Petén, Guatemala, showing localities mentioned in text.Chinajá.—Lat. 16° 02´, long. 90° 13´, elev. 140 m. Camp of the Ohio Oil Company of Guatemala and formerly a small settlement. On some maps Chinajá is located just to the north of the Alta Verapaz—El Petén boundary; recent surveys place the location just to the south of the imaginary line through the rainforest. Field work was conducted in the immediate vicinity of the camp, on the lower slopes of the Serrania de Chinajá, and at several sites to the northwest and north-northwest of Chinajá, where the forest was being cleared. The entire area supports rainforest.
La Libertad.—Lat. 16° 47´, long. 90° 07´, elev., 170 m. A town on the savannas in central El Petén; although we collected there in the rainy season, the specimens obtained on the savannas are not included in this report.
Paso Subín.—Lat. 16° 38´, long. 90° 12´, elev. 90 m. A small settlement on the Río Subín, a tributary of the Río de la Pasión. Specimens were obtained in rainforest in the immediate vicinity of the settlement.
Río de la Pasión.—A large river flowing northward through southern El Petén and thence westward into the Río Usumacinta. Specimens were obtained along the river between the Río Subín and Sayaxché.
Río San Román.—A river flowing northward in south-central El Petén to the Río Salinas (Usumacinta). We collected along the river at a place about 16 kilometers north-northwest of Chinajá, approximately at Lat. 16° 10´, long. 90° 17´, elev. 110 m. In the dry season the river was clear; it is surrounded by rainforest.
Sayaxché.—Lat. 16° 31´, long. 90° 09´, elev. 80 m. A town on the southern bank of the Río de la Pasión. Specimens were obtained in the rainforest and in cleared areas in the immediate vicinity of the town.
Toocog (formerly Sojío).—Lat. 16° 41´, long. 90° 02´, elev. 140 m. A camp of the Ohio Oil Company of Guatemala located at the rainforest-savanna edge, 15 kilometers southeast of La Libertad. Although we collected on the savannas as well as in the forest, especially to the east of the camp, only species obtained in the forest are considered in this report.
THE HERPETOFAUNA OF THE RAINFOREST
In presenting an account of the herpetofauna of southern El Petén three items need to be considered: (1) The composition of the fauna; (2) the ecology of the fauna; (3) the relationships of the fauna. Each of these topics is discussed briefly below. Logically a discussion of the origin of the fauna should follow, but this is being withheld for inclusion in a report on the herpetofauna of the entire El Petén by L. C. Stuart and the author; at that time the above topics will be expanded to cover the herpetofauna of the whole region.
Composition of the Fauna
Table 1.—Composition of the Herpetofauna in Southern El Petén, Guatemala.
|
Group |
Families | Genera | Species |
| Gymnophiona | (1)[A] | (1) | (1) |
| Caudata | 1 | 1 | 2 |
| Salientia | 6 | 10 (1) | 19 (1) |
| Crocodilia | 1 | 1 | 1 |
| Testudines | 4 | 7 | 8 |
| Sauria | 6 | 13 (1) | 19 (1) |
| Serpentes | 4 | 21 (7) | 29 (10) |
|
Total |
22 (1) | 53 (10) | 78 (13) |
[A] Numbers in parenthesis indicate the number of additional taxa that probably occur.
A total of 78 species of amphibians and reptiles has been found in the rainforests in southern El Petén; a break down into families and genera is given in table 1. Another 13 species probably occur in southern El Petén (see Hypothetical List of Species). The fauna primarily is composed of typical humid lowland forest inhabitants, such as:
| Hyla ebraccata | Eumeces sumichrasti |
| Hyla loquax | Ameiva festiva edwardsi |
| Phyllomedusa callidryas taylori | Imantodes cenchoa leucomelas |
| Smilisca phaeota cyanosticta | Leptophis ahaetulla praestans |
| Anolis biporcatus | Xenodon rabdocephalus mexicanus |
| Anolis capito | Bothrops nasutus |
| Anolis humilis uniformis | Bothrops schlegeli schlegeli |
Nevertheless, the region also provides at least a limited amount of habitat suitable for some species that are more frequently found in open forest of a drier nature; such species include:
| Hyla microcephala martini | Anolis sericeus sericeus |
| Hyla staufferi | Eumeces schwartzei |
| Hypopachus cuneus nigroreticulatus | Oxybelis aeneus aeneus |
Because of the absence of sufficiently open habitat or owing to the presence of competitors, some conspicuous members of sub-humid forests are not present in southern El Petén. Conspicuous absentees are the following:
| Rhinophrynus dorsalis | Ameiva undulata |
| Phrynohyas spilomma | Cnemidophorus angusticeps |
| Triprion petasatus | Conophis lineatus |
| Anolis tropidonotus | Masticophis mentovarius mentovarius |
| Ctenosaura similis |
PLATE 7
 Fig. 1. Edge of rainforest along airstrip at Chinajá, El Petén, Guatemala.
Fig. 1. Edge of rainforest along airstrip at Chinajá, El Petén, Guatemala. Fig. 2. Rainforest at edge of savanna at Toocog, El Petén, Guatemala.
Fig. 2. Rainforest at edge of savanna at Toocog, El Petén, Guatemala.PLATE 8
 Interior of rainforest at Chinajá. Notice size of buttresses on large tree (Ceiba pentandra).
Interior of rainforest at Chinajá. Notice size of buttresses on large tree (Ceiba pentandra).PLATE 9
 Interior of rainforest at Toocog. Notice less dense vegetation as compared with Pl. 8.
Interior of rainforest at Toocog. Notice less dense vegetation as compared with Pl. 8.PLATE 10
 Fig. 1. Rainforest along Río San Román, 16 kilometers north-northwest of Chinajá.
Fig. 1. Rainforest along Río San Román, 16 kilometers north-northwest of Chinajá. Fig. 2. Rain pond in forest at Toocog. This was a breeding site for six species of frogs.
Fig. 2. Rain pond in forest at Toocog. This was a breeding site for six species of frogs.Ecology of the Herpetofauna
Our two visits to Chinajá and Toocog afforded the opportunity to gather data on the ecology of the rainforests of southern El Petén and to study the relationships between the environment and members of the herpetofauna. Tropical rainforests present the optimum[213] conditions for life, and it is in this environment that life reaches its greatest diversity. Here, too, biological inter-relationships are most complex. This complexity is illustrated by the presence of many species of some genera, all of which are found together in the same geographic region. In the rainforests of southern El Petén there are six species of Anolis, five of Hyla, four of Bothrops, and three of Coniophanes. Obviously, the diversity of ecological niches in the rainforest is sufficient to support a variety of related species. Of the examples mentioned above, fairly adequate ecological data were obtained for most of the species of Anolis, which will be used to show the ecological diversity and vertical stratification of sympatric species in the rainforests.
Of the six species of Anolis, all except A. sericeus are typically found in humid forests. Anolis sericeus sericeus is poorly represented in the collections from southern El Petén, where it may be in competition with Anolis limifrons rodriguezi that resembles Anolis s. sericeus in size, coloration, and habits. Therefore, Anolis sericeus sericeus is excluded from the following discussion. The common terrestrial species is Anolis humilis uniformis; sometimes this small species perches or suns on the bases of small trees or buttresses of some large trees. When disturbed it takes to the ground and seeks cover in the leaf litter or beneath logs or palm fronds. Anolis lemurinus bourgeaei is about twice the size of Anolis humilis uniformis and is usually observed on buttresses of large trees or on the lower two meters of tree trunks. Individuals were seen foraging on the ground along with Anolis humilis uniformis. At no time were Anolis lemurinus bourgeaei observed to ascend the trunks of large trees; they always took refuge near the bases of trees. Anolis limifrons rodriguezi is found on the stems and branches of bushes. It is a small species that sometimes is observed on the ground but was never seen ascending large trees. Anolis capito is about the same size as Anolis lemurinus bourgeaei and lives on the trunks of large trees. In the tops of the trees lives a large green species, Anolis biporcatus.
Similar segregation habitatwise can be demonstrated for other members of the herpetofauna. The avoidance of interspecific competition in feeding is well illustrated by three species of snakes that probably are the primary ophidian predators on frogs. Drymobius margaritiferus margaritiferus is diurnal and terrestrial; it feeds on frogs at the edges of breeding ponds by day. Also during the day Leptophis mexicanus mexicanus feeds on frogs in[214] bushes and trees. At night the activities of both of these species is replaced by those of Leptodeira septentrionalis polysticta, which not only feeds on the frogs in the trees and bushes, but descends to the ground and even enters the water in search of food.
From the examples discussed above, the importance of the three dimensional aspect of the rainforest is apparent. The presence of a large and diverse habitat above the ground is of great significance in the rainforest, for of the non-aquatic components of the herpetofauna in the rainforests of southern El Petén, 42 per cent of the species spend at least part of their lives in the bushes and trees. Another important part of the forest is the subterranean level—the rich mulch, underground tunnels, and rotting subterranean vegetation. Of the 78 species of amphibians and reptiles in southern El Petén, seven are primarily fossorial, and half-a-dozen others are secondarily fossorial. Probably the fossorial members of the fauna are the least well represented in the collection, for such widespread species as Dermophis mexicanus mexicanus, Rhadinaea decorata decorata and Tantilla schistosa schistosa were expected, but not found.
In the following discussion of the ecological distribution of amphibians and reptiles in the rainforest I have depended chiefly on my observations made in southern El Petén, but have taken into consideration observations made on the same species in other regions, together with reports from other workers. The reader should keep in mind that the evidence varies from species to species. Of some species I have observed only one animal in the field; of others, I have seen scores and sometimes hundreds of individuals. For species on which I have few observations or rather inconclusive evidence, the circumstance of inadequate data is mentioned.
In analyzing the ecological distribution within the forest, it is convenient to recognize five subdivisions (habitats); each is treated below as a unit.
1. Aquatic.—This habitat includes permanent streams and rivers (Pl. 10, fig. 1), some of which are clear and others muddy. In the rainy season temporary ponds form in depressions on the forest floor (Pl. 10, fig. 2); these are important as breeding sites for many species of amphibians. Aquatic members of the herpetofauna are here considered to be those species that either spend the greatest part of their lives in the water or usually retreat to water for shelter. Seven species of turtles and one crocodilian are[215] aquatic. Of these, Dermatemys mawi, Staurotypus triporcatus, and Pseudemys scripta ornata inhabit clear water, whereas Chelydra rossignoni, Claudius angustatus, Kinosternon acutum, and K. leucostomum inhabit muddy water. Crocodylus moreleti apparently inhabits both clear and muddy water, for in the dry season it lives along the clear rivers, but in the rainy season inhabits flooded areas in the forest as well.
2. Aquatic Margin.—Extensive marshes were lacking in the part of southern El Petén that I visited; consequently, the aquatic margin habitat is there limited to the edges of rivers and borders of temporary ponds. Bufo marinus, Rana palmipes, and Rana pipiens are characteristic inhabitants of the aquatic margin, although in the rainy reason Bufo marinus often is found away from water. Observations indicate that Tretanorhinus nigroluteus lateralis inhabits the margins of ponds and streams and actually spends considerable time in the water. Although Iguana iguana rhinolopha is arboreal, it lives in trees along rivers, into which it plunges upon being disturbed. Species included in this category are those that customarily spend most of their lives at the edge of permanent water. Frogs and toads that migrate to the water for breeding and the snakes that prey on the frogs at that time are not assigned to the aquatic-margin habitat.
3. Fossorial.—Characteristic inhabitants of the mulch on the forest floor are Bolitoglossa moreleti mulleri, Lepidophyma flavimaculatum flavimaculatum, Scincella cherriei cherriei, Ninia sebae sebae, Pliocercus euryzonus aequalis, and Micrurus affinis apiatus. Other species of snakes that spend most of their lives above ground often forage in the mulch layer; among these are Coniophanes bipunctatus biserialis, Coniophanes fissidens fissidens, Coniophanes imperialis clavatus, Lampropeltis doliata polyzona, and Stenorrhina degenhardti. Among the amphibians, at least Hypopachus cuneus nigroreticulatus, Eleutherodactylus rostralis, and Syrrhophus leprus are known to seek shelter in the mulch.
4. Terrestrial.—One turtle, Geoemyda areolata, is primarily terrestrial. Among the lizards, conspicuous terrestrial species are Anolis humilis uniformis and Ameiva festiva edwardsi; Anolis lemurinus bourgeaei and Basiliscus vittatus spend part of their lives on the ground, but also live on trees and in bushes. Eumeces schwartzei and E. sumichrasti apparently are terrestrial. The only terrestrial lizard that is nocturnal is Coleonyx elegans elegans, which[216] by day hides in the leaf litter or below ground. Nocturnal amphibians that are terrestrial include Bufo marinus, Bufo valliceps valliceps, Eleutherodactylus rugulosus rugulosus, Syrrhophus leprus, and Hypopachus cuneus nigroreticulatus. A large number of active diurnal snakes are terrestrial; these include Boa constrictor imperator, Clelia clelia clelia, Dryadophis melanolomus laevis, Drymarchon corais melanurus, Drymobius margaritiferus margaritiferus, Pseustes poecilonotus poecilonotus, and Spilotes pullatus mexicanus. Nocturnal terrestrial snakes include three kinds of Bothrops (B. atrox asper, B. nasutus, and B. nummifer nummifer), all of which seem to be equally active by day.
5. Arboreal.—In this habitat the third dimension (height) of the rainforest probably is the most complex insofar as the inter-relationships of species and ecological niches are concerned. I have attempted to categorize species as to microhabitats within the arboreal habitat; in so doing, I recognize four subdivisions—bushes, tree trunks, tree tops, and epiphytes.
Bush inhabitants include several species of lizards and snakes, all of which have rather elongate, slender bodies, and long tails. Common bush-inhabitants in southern El Petén are Anolis limifrons rodriguezi, Basiliscus vittatus, Laemanctus deborrei, Leptophis mexicanus mexicanus, and Oxybelis aeneus aeneus. All of these are diurnal, and all but Laemanctus have been observed sleeping on bushes at night.
Tree-trunk inhabitants include five species of lizards. Thecadactylus rapicaudus lives on the trunks of large trees; Sphaerodactylus lineolatus lives beneath the bark on dead trees and on corozo palms. Anolis lemurinus bourgeaei lives on the bases and buttresses of large trees, from which it often descends to the ground. Corythophanes cristatus and Anolis capito were found only on tree trunks and large vines.
The least information is available for the species living in the tree tops. The following species were obtained from tops of trees when they were felled, or have been observed living in the tree tops: Anolis biporcatus, Iguana iguana rhinolopha, Celestus rozellae, Leptodeira septentrionalis polysticta, Leptophis ahaetulla praestans, Sibon dimidiata dimidiata, and Sibon nebulata nebulata.
Epiphytes, especially the bromeliads, provide refuge for a variety of tree frogs and small snakes. Of the tree frogs, Hyla picta, Hyla staufferi, Phyllomedusa callidryas taylori, Similisca baudini, and Similisca phaeota cyanosticta have been found in bromeliads; other[217] species probably occur there. Among the snakes, Imantodes cenchoa leucomelas, Leptodeira frenata malleisi, Leptodeira septentrionalis polysticta, Sibon dimidiata dimidiata, and Sibon nebulata nebulata are frequent inhabitants of bromeliads; all of these snakes are nocturnal.
Relationships of the Fauna
Most of the 78 species of amphibians and reptiles definitely known from the rainforest in southern El Petén have extensive ranges in the Atlantic lowlands of southern México and Central America; many extend into South America. Sixty-two (80%) of the species belong to this group having extensive ranges in Middle America. Three species (Syrrhophus leprus, Leptodeira frenata, and Kinosternon acutum) are at the southern limits of their distributions in southern El Petén and northern Alta Verapaz, whereas Eleutherodactylus rostralis and Thecadactylus rapicaudus are at the northern and western limits of their distributions in El Petén. Nine (11%) species have the center of their distributions in El Petén and the Yucatán Peninsula; representatives of this group include Claudius angustatus, Dermatemys mawi, Laemanctus deborrei, and Eumeces schwartzei.
In determining a measure of faunal resemblance, I have departed from the formulae discussed by Simpson (1960) and have analyzed the degree of resemblance by the following formula used to calculate an index of faunal relationships:
C (2) / (N1 + N2) = R, where
C = species common to both faunas.
N1 = number of species in the first fauna.
N2 = number of species in the second fauna.
R = degree of relationships (when R = 1.00, the faunas are identical; when R = 0, the faunas are completely different).
The herpetofauna of southern El Petén has been compared with that in the Tikal-Uaxactún area (Stuart, 1958), that in the humid lowlands of Alta Verapaz (Stuart, 1950, plus additional data), and that in the Mexican state of Yucatán (Smith and Taylor, 1945, 1948, and 1950). The herpetofaunas of lowland Alta Verapaz and Yucatán are the largest, having respectively 94 and 91 species, where as there are 78 species known from southern El Petén and 64 from the Tikal-Uaxactún area. An analysis of faunal relationships (Table 2) shows that the faunas of the rainforests of southern El Petén and lowland Alta Verapaz are closely related. The relationships between these two areas and the Tikal-Uaxactún area[218] in northern El Petén is notably less. Apparently the biggest faunal changes take place between southern El Petén and the Tikal-Uaxactún area, and between the latter and Yucatán. As stated by Stuart (1958:7) the Tikal-Uaxactún is transitional between the humid rainforests to the south and the dry outer end of the Yucatán Peninsula. The transitional nature of the environment is exemplified by a rather depauperate herpetofauna consisting of some species of both dry and humid environments and lacking a large fauna typical of either. Contrariwise, the continuity of the environment from southern El Petén to the lowlands of Alta Verapaz is reflected in degree of resemblance of the herpetofaunas.
Table 2.—Index of Faunal Relationships Between Southern El Petén and Other Regions.
| Lowland Alta Verapaz |
Southern El Petén |
Tikal- Uaxactún Area |
Yucatán | |
| Lowland Alta Verapaz | .85 | .61 | .43 | |
| Southern El Petén | .85 | .64 | .41 | |
| Tikal-Uaxactún Area | .61 | .64 | .63 | |
| Yucatán | .43 | .41 | .63 |
Most of the species of amphibians and reptiles found in southern El Petén are found in humid tropical forests from the Isthmus of Tehuantepec southeastward on the Atlantic lowlands well into Central America.
ACCOUNTS OF SPECIES
In the following pages various aspects of the occurrence, life histories, ecology, and variation of the species of amphibians and reptiles known from southern El Petén are discussed. Only Cochranella fleischmanni reported by Stuart (1937) from Río Subín at Santa Teresa was not collected by us and is excluded. Because more worthwhile information was gathered for some species than others, the length and completeness of the accounts vary. All specimens listed are in the Museum of Natural History at the University of Kansas, to which institution all catalog numbers refer. Preceding the discussion of each species is an alphabetical list of[219] localities from which specimens were obtained; numbers after a locality indicate the number of specimens obtained at each locality.
Bolitoglossa dofleini (Werner)
Chinajá, 1.
An adult female having minute ovarian eggs has a snout-vent length of 81 mm., a tail length of 59 mm., 13 costal grooves, two intercostal spaces between adpressed toes, 38-35 vomerine teeth in irregular rows forming a broad arch from a point posterolaterad to the internal nares to a point near the anterior edge of the parasphenoid teeth, and 43-44 maxilliary-premaxillary teeth. In life the dorsum was rusty brown with irregular black and orange spots and streaks. The flanks were bluish gray with black in the costal grooves and creamy tan flecks along the ventral edge of the flank. The belly and underside of the tail were yellowish tan with dark brown spots laterally. The limbs were orange proximally and black distally; the pads of the feet were bluish black. The dorsal and lateral surfaces of the tail were yellowish orange with black spots. The iris was grayish yellow.
Stuart (1943:17) reported this species from Finca Volcán, Alta Verapaz. He diagnosed his specimens as having 13 costal grooves and two or three intercostal spaces between adpressed toes. He stated that the vomerine teeth were about 12 in number and that in life the dorsum was mottled gray and black, the sides gray and brown, and the undersurfaces uniformly dark gray. These specimens differ noticeably from the individual from Chinajá in the number of vomerine teeth and in coloration.
In August, 1961, I obtained a specimen of Bolitoglossa dofleini at Finca Los Alpes, Alta Verapaz, approximately 13 kilometers airline south-southwest of Finca Volcán and at approximately the same elevation. Although the salamander was dead when found, it obviously was more heavily pigmented than the individual from Chinajá. The belly was bluish gray with black spots laterally; the dorsum was dull brownish gray with some brownish red streaks. The specimen is a female having small ovarian eggs, a snout-vent length of 90 mm., 13 costal grooves, and two intercostal spaces between adpressed limbs. There are 28-29 vomerine teeth, more than twice as many as in specimens from Finca Volcán (Stuart, 1943:17), but noticeably fewer than in the specimen from Chinajá.
The presence of this species at Chinajá lends support to the idea that the specimen from the Río de la Pasión listed by Brocchi[220] (1882:116) also is Bolitoglossa dofleini. Furthermore, the confirmed presence of this species in the lowlands of El Petén suggests that there may be genetic connection between B. dofleini in the Alta Verapaz and B. yucatana in the Yucatán Peninsula. Bolitoglossa yucatana differs from B. dofleini in having five intercostal spaces between adpressed toes and in having a different color pattern. Both are robust species having no close relationships to other species of Bolitoglossa in northern Central America.
The specimen from Chinajá was found in water in the axil of a large elephant-ear plant (Xanthosoma) by day in March. Its stomach contained fragments of beetles and a large roach. The natives did not know salamanders and had no name for them.
Bolitoglossa moreleti mulleri (Brocchi)
Chinajá, 2; Río San Román, 1.
One specimen is a female having a snout-vent length of 80 mm., a tail length of 82 mm., and a total length of 162 mm. It contains 63 large eggs, the largest of which has a diameter of about three millimeters. This specimen has 13 costal grooves, four intercostal spaces between adpressed toes, and 12-13 vomerine teeth. A juvenile having a snout-vent length of 39 mm. and a tail length of 33 mm. has 12 costal grooves, three intercostal spaces between adpressed toes, and 8-8 vomerine teeth. In life these salamanders were uniformly dull brownish black above with a dull creamy yellow irregular dorsal stripe beginning on the occiput and continuing onto the tail. There are no yellow or orange streaks or flecks on the head or limbs. The specimen from the Río San Román was taken from the stomach of a Pliocercus euryzonus aequalis and has not been studied in detail, because of its poor condition.
The present specimens show no tendency for the development of a broad irregular dorsal band that encloses black spots or forms irregular dorsolateral stripes, as is characteristic of B. moreleti mexicanus, a subspecies that has been reported from La Libertad (Stuart, 1935:35) and Piedras Negras (Taylor and Smith, 1945:545) in El Petén, and from Xunantunich, British Honduras (Neill and Allen, 1959:20).
Schmidt (1936:151) and Stuart (1943:13) found B. moreleti mulleri in bromeliads at Finca Samac, Alta Verapaz. Taylor and Smith's (1945:545) and Neill and Allen's (1959:20) specimens of B. moreleti mexicanus were obtained from bromeliads, but Neill and Allen (loc. cit.) stated that the natives in British Honduras[221] said that they had found salamanders beneath rubbish on the forest floor. My specimens were obtained from beneath logs on the forest floor in the rainy season. Possibly in drier environments the species characteristically inhabits bromeliads, at least in the dry season.
Bufo marinus (Linnaeus)
Chinajá, 3; 10 km. NNW of Chinajá, 1; 11 km. NNW of Chinajá, 1.
During both visits to Chinajá this large toad was breeding in a small permanent pond in the camp. During the day the toads took refuge in crevices beneath the buildings or beneath large boulders by the pond. At dusk from four to ten males congregated at the pond and called. Tadpoles of this species were in the pond in March and in July. One juvenile was found beneath a rock in the forest, and another was on the forest floor by day.
The natives' name for this species and the following one is sapo.
Bufo valliceps valliceps Wiegmann
Chinajá, 52; Río San Román, 8; Sayaxché, 2; Toocog, 1.
This is one of the most abundant, or at least conspicuous, amphibians inhabiting the forest. Breeding congregations were found on February 24, March 2, March 11, and June 27. At these times the toads were congregated at temporary ponds in the forest or along small sluggish streams. Throughout the duration of both visits to Chinajá individual males called almost nightly at the permanent pond at the camp.
The variation in snout-vent length of 20 males selected at random is 56.7 to 72.5 mm. (average, 64.8 mm.). Two adult females have snout-vent lengths of 80.4 and 87.6 mm. In all specimens the parotid glands are somewhat elongated and not rounded as in Bufo valliceps wilsoni (see Baylor and Stuart, 1961:199). My observations on the condition of the cranial crests of the toads in El Petén agree with the findings of Baylor and Stuart (op. cit.:198) in that hypertrophied crests are usual in large females. In the shape of the parotids and nature of the cranial crests the specimens from El Petén are like those from the Isthmus of Tehuantepec in México. As I pointed out (1960:53), the validity of the subspecies Bufo valliceps macrocristatus, described from northern Chiapas by Firschein and Smith (1957:219) and supposedly characterized by hypertrophied cranial crests, is highly doubtful.
In the toads from El Petén the greatest variation is in coloration. The dorsal ground-color varies from orange and rusty tan to brown, yellowish tan, and pale gray. In some individuals the[222] flanks and dorsum are one continuous color, whereas in others a distinct dorsolateral pale colored band separates the dorsal color from dark brown flanks. In some individuals the venter is uniform cream color, in others it bears a few scattered black spots, and in still others there are many spots, some of which are fused to form a black blotch on the chest. In breeding males the vocal sac is orange tan. All specimens have a coppery red iris.
Aside from the breeding congregations, active toads were found on the forest floor at night; a few were there by day. Some individuals were beneath logs during the day.
Eleutherodactylus rostralis (Werner)
Chinajá, 10.
Because of the multiplicity of names and the variation in coloration, the small terrestrial Eleutherodactylus in southern México and northern Central America are in a state of taxonomic confusion. Stuart (1934:7, 1935:37, and 1958:17) referred specimens from El Petén to Eleutherodactylus rhodopis (Cope). Stuart (1941b:197) described Eleutherodactylus anzuetoi from Alta Verapaz and El Quiché, Guatemala, suggested that the new species was an upland relative of Eleutherodactylus rostralis (Werner), and used that name for the frogs that he earlier had referred to Eleutherodactylus rhodopis. Dunn and Emlen (1932:24) placed E. rostralis in the synonymy of E. gollmeri (Peters). Examination of series of these frogs from southern México, Guatemala, and Costa Rica causes me to think that there are four species; these can be distinguished as follows:
E. rhodopis.—No web between toes; one tarsal tubercle; tibiotarsal articulation reaches to nostril; iris bronze in life.
E. anzuetoi.—No web between toes; a row of tarsal tubercles; tibiotarsal articulation reaches to tip of snout; color of iris unknown.
E. rostralis.—A vestige of web between toes; no tarsal tubercles; tibiotarsal articulation reaches snout or slightly beyond; iris coppery red in life.
E. gollmeri.—A vestige of web between toes; no tarsal tubercles; tibiotarsal articulation reaches well beyond snout; iris coppery red in life.
The presence of webbing between the toes, the absence of tarsal tubercles, and the coppery red iris distinguish E. rostralis and E. gollmeri from the other species. Probably E. rostralis and E. gollmeri are conspecific, but additional specimens are needed from Nicaragua and Honduras to prove conspecificity. On the other hand, the characters of the frogs from Chinajá clearly show that they are related to E. gollmeri to the south and not to E. rhodopis to the north in México.
At Chinajá, Eleutherodactylus rostralis was more abundant than[223] the few specimens indicate, for upon being approached the frogs moved quickly and erratically, soon disappearing in the leaf litter on the forest floor. Most of the specimens were seen actively moving on the forest floor in the daytime; one was found beneath a rock, and one was on the forest floor at night.
Eleutherodactylus rugulosus rugulosus (Cope)
Chinajá, 2; 15 km. NW of Chinajá, 4.
These frogs were found on the forest floor by day. With the exception of one female having a snout-vent length of 69.5 mm., all are juveniles. The apparent rarity of this species at Chinajá may be due to the absence of rocky streams, a favorite habitat of this frog. The local name for this frog is sapito, meaning little toad.
Leptodactylus labialis (Cope)
Toocog, 1.
One juvenile having a snout-vent length of 16.4 mm. was found at night beside a pond in the forest. The scarcity of the species of Leptodactylus in the southern part of El Petén probably is due to the lack of permanent marshy ponds.
Leptodactylus melanonotus (Hallowell)
Sayaxché, 1.
One individual was found beneath a rock beside a stream in the forest. The local name is ranita, meaning little frog.
Syrrhophus leprus Cope
Chinajá, 2; 15 km NW of Chinajá, 1.
An adult female having a snout-vent length of 27.5 mm. was found on the forest floor by day. Two juveniles having snout-vent lengths of 15.5 and 19.0 mm. were beneath rocks on the forest floor. The specimens are typical of the species as defined by Duellman (1958:8).
Hyla ebraccata Cope
Toocog, 66.
This small tree frog congregated in large numbers at a forest pond at Toocog. Between June 30 and July 2 we collected specimens and observed the breeding habits of this and other species at the pond. Calling males were distributed around the pond, where they called from low herbaceous vegetation at the edge of the pond or from plants rising above the water. Calling commenced at dusk[224] and continued at least into the early hours of the morning. On one occasion a female was observed at a distance of about 50 centimeters away from a calling male sitting on a blade of grass. The female climbed another blade of grass until she was about eight centimeters away from the male, at which time he saw her, stopped calling, jumped to the blade of grass on which she was sitting and clasped her. Clasping pairs were observed on blades of grass and leaves of plants above the water; most pairs were less than 50 centimeters above the surface of the pond.
The eggs are deposited on the dorsal surfaces of leaves above the water. All eggs are in one plane (a single layer) on the leaf. External membranes are barely visible, as the eggs consist of a single coherent mass. Eggs in the yolk plug stage have diameters of 1.2 to 1.4 mm. Seventeen eggs masses were found; these contained from 24 to 76 (average 44) eggs. The jelly is extremely viscous and tacky to the touch. At time of hatching the jelly becomes less viscous; the tadpoles wriggle until they reach the edge of the leaf and drop into the water.
Eleven tadpoles were preserved as they hatched; these have total lengths of 4.5 to 5.0 (average 4.77) mm. Hatchling tadpoles are active swimmers and have only a small amount of yolk. The largest tadpoles preserved have total lengths of 13.0 and 13.5 mm. At this size distinctive sword-tail and bright coloration have developed.
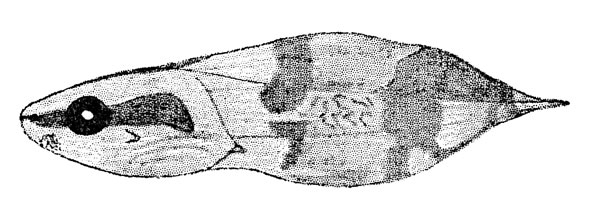 Fig. 2. Tadpole of Hyla ebraccata (KU 59986) from Toocog, El Petén, Guatemala.
Fig. 2. Tadpole of Hyla ebraccata (KU 59986) from Toocog, El Petén, Guatemala.Description of fully developed tadpole (KU 59986): Total length, 13.5 mm.; tail-length, 8.4 mm., 62 per cent of total length. Snout, in dorsal view, bluntly rounded; in lateral view less bluntly rounded; body depressed; head flattened; mouth terminal; eye large, its diameter 25 per cent of length of body; nostrils near tip of snout and directed anteriorly; spiracle sinistral and situated postero-ventrad to eye; cloaca median. Tail-fin thrice depth of tail-musculature, which extends beyond posterior end of tail-fin giving sword-tail appearance (Fig. 2). In life, black stripe on each side of body and on top of head; black band on anterior part of tail[225] and another on the posterior part; body and anterior part of tail creamy yellow; dark red band between black bands on tail. Mouth terminal, small, its width about one-fifth width of body; fleshy ridge dorsally and ventrally; row of small papillae on ventral lip; no lateral indentations of lips; upper beak massive, convex, and finely serrate; lower beak small and mostly concealed behind upper; no teeth (Fig. 3).
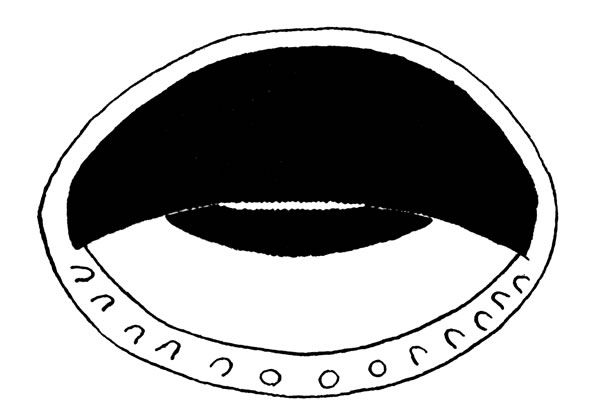 Fig. 3. Mouthparts of larval Hyla ebraccata (KU 59986) from Toocog, El Petén, Guatemala.
Fig. 3. Mouthparts of larval Hyla ebraccata (KU 59986) from Toocog, El Petén, Guatemala.
Hyla loquax Gaige and Stuart
Toocog, 14.
These specimens were found at night when they were calling from low vegetation in a forest pond. Most of the frogs were several meters away from the edge of the pond. Although two clasping pairs were found, we obtained no eggs or tadpoles referable to this species.
Hyla microcephala martini Smith
Chinajá, 1; Toocog, 21.
The specimen from Chinajá was calling from a small bush at the edge of a temporary grassy pond in a clearing in the forest. At Toocog this species was closely associated with Hyla ebraccata; males were calling from herbaceous vegetation in and around the forest pond. These frogs were not so abundant in the forest at Toocog as they were around ponds on the savanna at La Libertad.
Hyla picta (Günther)
Toocog, 8.
This small tree frog was calling from herbs in a pond in the forest on June 30 and July 2. The voice is weak; probably greater numbers of males were present than are indicated by the few specimens collected, for the din from the more vociferous species made it impossible to hear Hyla picta unless one was calling close by.
Hyla staufferi Cope
Chinajá, 1.
This individual was calling from a low bush in the clearing at Chinajá. None was found in the pond in the forest at Toocog. Stuart (1935:38) and Duellman (1960:63) noted that Hyla staufferi breeds early in the rainy season. Nevertheless, I think early breeding habits do not account for the near absence of this species in our collections from southern El Petén. In early July, 1960, a few individuals were heard at a pond on the savanna at La Libertad. In mid-July of the same year they were calling sporadically from temporary ponds in the lower Motagua Valley. Possibly the individual collected at Chinajá was accidentally transported there in cargo from Toocog, from which camp at the edge of the savanna planes fly to Chinajá weekly. My observations on this species throughout its range in México and Central America indicate that it inhabits savannas and semi-arid forests and usually is absent from heavy rainforest. Stuart (1948:34) obtained this species at Cubilquitz in the lowlands of Alta Verapaz.
Phyllomedusa callidryas taylori Funkhouser
Toocog, 25.
Between June 30 and July 2 this species was abundant at a pond in the forest at Toocog. Calling males were as high as five meters in bushes and trees around the pond. At dusk males were observed descending a vine-covered tree at the edge of the pond; this strongly suggests that the frogs retreat to this tree and others like it for diurnal seclusion. Clasping pairs were found on branches and leaves above the water. The eggs are deposited in clumps usually on vertical leaves, but sometimes on horizontal leaves or on branches, vines, and aerial roots above the water. Twenty-six clutches of eggs contained from 14 to 44 (average 29) eggs. In a clutch in which the eggs are in yolk plug stage the average diameter of the embryos is 2.3 mm. and that of the vitelline[227] membranes, 3.4 mm. Most of the eggs are in the external part of the gelatinous mass; the jelly is clear. The yolk is pale green, and the animal pole is brown. As development ensues, the yolk becomes yellow and the embryo first dark brown and then pale grayish tan. Upon hatching the tadpoles wriggle free of the jelly and drop into the water. One clutch of 19 eggs was observed to hatch in three minutes. Apparently, on dropping into the water the hatchling tadpoles go to the bottom of the pond, for one or two minutes pass from the time they enter the water until they reappear near the surface. The average total length of seven hatchling tadpoles is 7.4 mm. There is a moderate amount of yolk, but this does not form a large ventral bulge. Large tadpoles congregate in the sunny parts of the pond, where they were observed just beneath the surface. Many had their mouths at the surface. Except for constant fluttering of the tip of the tail, they lie quietly with the axis of the body at an angle of about 45 degrees with the surface of the water.
Description of tadpole (KU 60006): total length, 24.5 mm.; tail-length, 15.4 mm.; body broader than deep; head moderately flattened; snout viewed from above blunt; nostrils close to snout and directed dorsally; eyes of moderate size and directed laterally; mouth directed anteroventrally; anus median; spiracle ventral, its opening just to left of midline slightly more than one-half distance from tip of snout to vent. Tail-fin slightly more than twice as deep as tail musculature, which curves upward posteriorly; tail-fin narrowly extending to tip of tail (Fig. 4). Color in life pale gray; in preservative white with scattered melanophores; tail-fin transparent.
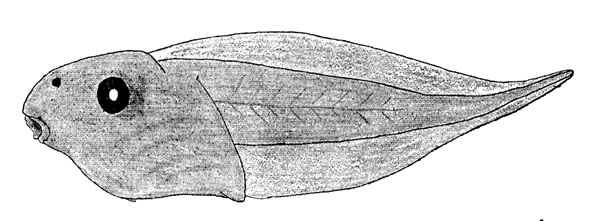 Fig. 4. Tadpole of Phyllomedusa callidryas taylori (KU 60006) from Toocog, El Petén, Guatemala.
Fig. 4. Tadpole of Phyllomedusa callidryas taylori (KU 60006) from Toocog, El Petén, Guatemala.Upper lip having single row of papillae laterally, but none medially; lower lip having single row of papillae; no lateral indentation of lips; two or more rows of papillae at lateral corners of lips; tooth-rows 2/3; second upper tooth row as long as first, interrupted medially; inner lower tooth-row as long as upper rows,[228] interrupted medially; second and third lower rows decreasingly shorter; upper beak moderate in size and having long lateral projections; lower beak moderate in size; both beaks finely serrate (Fig. 5).
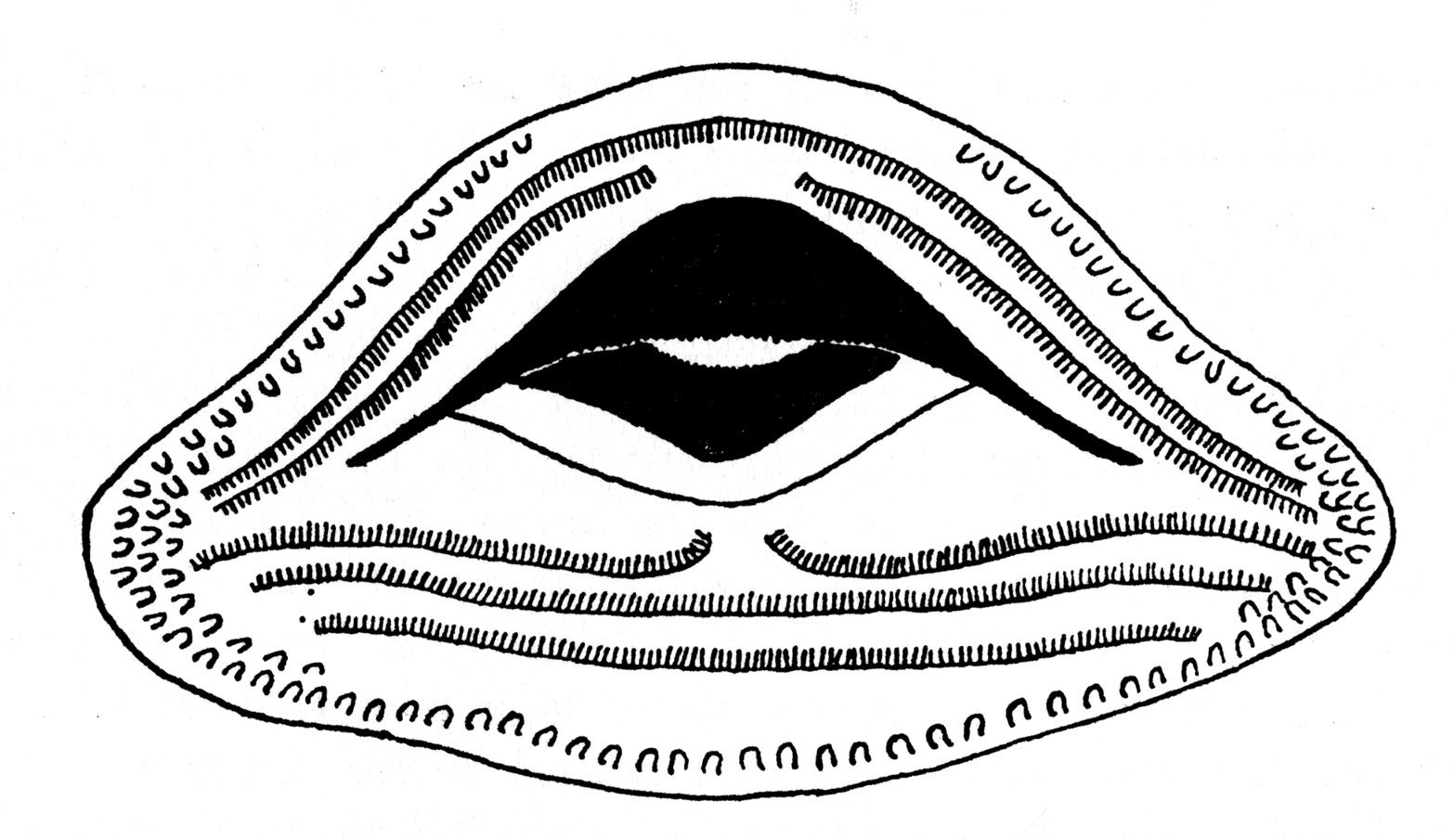 Fig. 5. Mouthparts of larval Phyllomedusa callidryas taylori (KU 60006) from Toocog, El Petén, Guatemala.
Fig. 5. Mouthparts of larval Phyllomedusa callidryas taylori (KU 60006) from Toocog, El Petén, Guatemala.
Smilisca baudini (Duméril and Bibron)
Chinajá, 9; 20 km. NNW of Chinajá, 42; Río de la Pasión, 1; Río San Román, 5; Sayaxché; Toocog, 2.
Individuals of this species were found at night sitting on bushes and small trees in the forest in February and March and again in June and July. One was in the axil of a leaf of a Xanthosoma. In June and July males were heard nearly every night. The series of specimens from 20 kilometers north-northwest of Chinajá was taken from a breeding congregation in a shallow muddy pool in the forest. Tadpoles of this species were in small, often muddy pools in the forest. To my knowledge Smilisca baudini is the only hylid to breed in these pools at Chinajá, although perhaps Smilisca phaeota also utilizes them. The only other amphibian at Chinajá known to breed in the pools is Bufo valliceps valliceps. Although two specimens were on bushes at night at Toocog, Smilisca baudini was not present at the pond where five other species of hylids were breeding. Nevertheless, Smilisca baudini was calling from two ponds on the savannas near La Libertad. All of the specimens from southern El Petén have yellow or yellowish white flanks and ventrolateral surfaces.
Smilisca phaeota cyanosticta (Smith)
Chinajá, 4; 10 km. NNW of Chinajá, 1.
All specimens were found in February and March. Those from Chinajá were obtained from Xanthosoma and bromeliads; the individual from 10 kilometers north-northwest of Chinajá is an adult male that was calling from a puddle in a fallen tree on March 13. A juvenile having a snout-vent length of 34.7 mm. lacks the pale blue spots on the thighs; instead, the anterior and posterior surfaces of the thighs are bright red.
Hypopachus cuneus nigroreticulatus Taylor
Toocog, 1.
An adult male having a snout-vent length of 41.7 mm. was found at night on the forest floor at the edge of a temporary pond. In life the dorsum was dark brown with chocolate brown markings; the stripe on the side of the head was white; the middorsal stripe was pale orange; the belly was black and white, and the iris was a bronze color.
Characteristically this species inhabits savannas and open forest; thus, its occurrence in the rainforest at Toocog is surprising. This is the southernmost record for the species in El Petén; to the south in the highlands it is replaced by the smaller Hypopachus inguinalis, having rounded, instead of compressed, metatarsal tubercles.
Rana palmipes Spix
Chinajá, 11; 15 km. NW of Chinajá, 1; 20 km. NNW of Chinajá, 1.
With the exception of one recently metamorphosed juvenile having a snout-vent length of 30.7 mm. that was found on the forest floor by day on June 24, and one that was found beside a pool in a cave, all individuals were found at temporary woodland pools or along sluggish streams at night. The largest specimen is a female having a snout-vent length of 107 mm.
Rana pipiens Schreber
Chinajá, 1; 20 km. NNW of Chinajá, 1; Río San Román, 1; Toocog, 1.
All specimens were found near water at night. The largest individual is a female having a snout-vent length of 112.5 mm.
Crocodylus moreleti Duméril and Duméril
Chinajá, 1; Río San Román, 1.
One specimen was obtained from a quiet pool in the Río San[230] Román at night; another was found in a small sluggish stream at Chinajá. Two large individuals were seen in tributaries to the Río San Román. On the savannas at Toocog two small individuals were obtained in the dry season, at which time the crocodiles apparently were migrating to water. The local name for this species is lagarto.
Chelydra rossignoni (Bocourt)
Chinajá, 1; 20 km. NNW of Chinajá, 1.
The paucity of specimens of Chelydra from Central America has resulted in rather inadequate diagnoses of various populations. The present specimens have carapace lengths of 250 and 238 mm. and plastral lengths of 185 and 176 mm. The length of carapace/bridge ratio is 6.0 and 6.1 per cent. Each individual has four barbels, the median pair of which are extremely long. In KU 55977 the lateral pair of barbels is forked at the base. The relative length of the plastral bridge in these specimens compares favorable with the ratio (.06-.08) given by Schmidt (1946:4) for five specimens from Honduras. Chelydra serpentina, which may occur sympatrically with C. rossignoni in some parts of Central America, has a narrower plastral bridge and only two barbels beneath the chin. Furthermore, C. rossignoni and C. osceola in Florida have long, flat tubercles on the dorsal and lateral surfaces of the neck, whereas C. serpentina has short, round tubercles.
The specimen from Chinajá was found in a small sluggish stream; the other individual was in a muddy pool in the forest. The local name is sambodanga.
Claudius angustatus Cope
20 km. NNW of Chinajá, 1.
One specimen was unearthed from the bank of a small muddy stream by a bulldozer. This individual represents the second record for the species in Guatemala; the first was provided by specimens, likewise found in muddy waters, at Tikal (Stuart, 1958:19). The local name is caiman.
Kinosternon acutum Gray
20 km. NNW of Chinajá, 4; 30 km. NNW of Chinajá, 2.
These turtles were found on the forest floor, in small sluggish streams, and in pools in the forest. One adult male had, in life, the top of the head yellow with black spots; the stripes on the head and neck were red. Specimens were obtained both in the dry[231] and rainy seasons. The local name for both species of Kinosternon is pochitoque.
Kinosternon leucostomum Duméril and Bibron
Chinajá, 3; 15 km. NW of Chinajá, 1; 20 km. NNW of Chinajá, 2.
Individuals of this turtle were found on the forest floor and in small sluggish streams. In life most specimens had a tan or pale brown head with pinkish tan stripes on the head and neck. All individuals were obtained in February and March. No ecological differences between this species and K. acutum were evident.
Staurotypus triporcatus (Wiegmann)
Paso Subín, 1.
This species is represented in the collection by one complete shell found on the bank of the Río Subín. The carapace has a length of 292 mm. The local name is Guao. Natives stated that this turtle was not uncommon in clear rivers and lakes, a habitat suggested for the species by Stuart (1958:19).
Dermatemys mawi Gray
Chinajá, 1; Río San Román, 4.
The record from Chinajá is based on a carapace found in a chiclero camp, where the turtle evidently had been brought for food. The four specimens from the Río San Román were obtained from edges of deep pools in clear water. In adult males the top of the head was reddish orange in life. One of the specimens from the Río San Román currently is living in the Philadelphia Zoological Gardens. The local name for this turtle is tortuga blanca; it is sought for its meat.
Geoemyda areolata (Duméril and Bibron)
Chinajá, 2.
Two specimens were obtained from dense forest at Chinajá. The local name is mojina.
Pseudemys scripta ornata (Gray)
Paso Subín, 1.
One subadult was obtained from clear water in the Río Subín. The stripes on the head and neck were yellow; there was no red "ear" on the side of the head. The stripes on the forelimbs were orange, and the ocelli on the carapace were red. The local name is jicotea.
Coleonyx elegans elegans Gray
Toocog, 1.
One adult male having a snout-vent length of 89 mm. was found beneath a log in the forest. Locally this gecko is known as escorpión; the natives believe it to be deadly poisonous. The use of the name escorpión seems to be restricted to lizards thought to be venomous. Nearly everywhere in México and Central America some species of lizard carries this appellation. In El Petén I heard the name used only for Coleonyx elegans and Thecadactylus rapicaudus; in the lowlands of Guerrero, México, the name is applied to geckos of the genus Phyllodactylus. The venomous lizards of the genus Heloderma in the lowlands of western México are called escorpiónes. In the mountains of southern México various skinks of the genus Eumeces, as well as lizards of the genus Xenosaurus, carry the same appellation. Abronia in the mountains of México and Gerrhonontus throughout México and Central America likewise are called escorpiónes. Although many people in various parts of Middle America consider most lizards poisonous, there is a unanimity of opinion concerning the venomous qualities of the various kinds of escorpiónes. I know of only two other lizards in Middle America that are so uniformly regarded in native beliefs; these are Enyaliosaurus clarki in the Tepalcatepec Valley in Michoacán, called nopiche, and Phrynosoma asio in western México, called cameleón.
Sphaerodactylus lineolatus Lichtenstein
15 km. NW of Chinajá, 1; Toocog, 1.
These small geckos were much more abundant than the few specimens indicate. They frequently were seen on the trunks of corozo palms, where they quickly took refuge in crevices at the bases of the fronds. The specimen obtained at Toocog was under the bark of a standing dead tree. In life the ventral surface of the tail was orange. The individual from Chinajá was in the leaf litter on the ground at the base of a dead tree.
Thecadactylus rapicaudus (Houttuyn)
15 km. NW of Chinajá, 1; 20 km. NNW of Chinajá, 2.
Two specimens were found beneath the bark of standing dead trees; another was found in the crack in the trunk of a mahogany tree about 13 meters above the ground. In life the dorsum was[233] yellowish tan with dark brown markings; the venter was yellowish tan with brown flecks, and the iris was olive-tan. The largest specimen is a male having a snout-vent length of 95 mm.; all specimens have regenerated tails. Individuals when caught twisted their bodies and attempted to bite; upon grabbing a finger they held on with great tenacity.
Anolis biporcatus (Wiegmann)
14 km. NNW of Chinajá, 1; 17 km. NNW of Chinajá, 1; 20 km. NNW of Chinajá, 3; 30 km. NNW of Chinajá, 1; Sayaxché, 1.
All specimens of this large anole were obtained from trees. Some individuals were found in the tops of trees immediately after they were felled. My limited observations on this anole suggest that it is an inhabitant of the upper levels of the forest. In life an adult male from 20 kilometers north-northwest of Chinajá was brilliant green above; the eyelids were bright yellow; the belly was white. The outer part of the dewlap was pale orange, and the median part was pinkish blue. A juvenile having a snout-vent length of 47 mm. and a tail length of 86 mm. was pale grayish green with pale gray flecks on the dorsum. The largest male has a snout-vent length of 98 mm. and a tail length of 217 mm.; the same measurements of the largest female are 89 and 213 mm. This species, together with all other anoles, is known locally as toloque.
Anolis capito Peters
Chinajá, 2; 14 km. NNW of Chinajá, 1; Río de la Pasión, 1.
All individuals were observed on trunks of trees between heights of three and ten meters above the ground. The largest male has a snout-vent length of 81 mm. and a tail length of 155 mm.; the same measurements of the largest female are 87 and 150 mm. The streaked brown dorsum, combined with the lizards' habit of pressing the body against the trunks of trees, make this anole especially difficult to see.
Anolis humilis uniformis Cope
Chinajá, 24; 15 km. NW of Chinajá, 22; 20 km. NNW of Chinajá, 6; Sayaxché, 1.
This small dull brown anole is a characteristic inhabitant of the forest floor, where the lizards move about in a series of quick, short hops and thus easily evade capture. Three individuals were found on small bushes, and four were on the bases of trees; otherwise, all were observed on the ground. Observations indicate that[234] this species is active throughout the day, except during and immediately after heavy rains. The males have a deep red dewlap with a dark blue median spot.
Anolis lemurinus bourgeaei Bocourt
Chinajá, 11; 20 km. NNW of Chinajá, 4; 30 km. NNW of Chinajá, 2; Río de la Pasión, 1; Río San Román, 1; Sayaxché, 8; Toocog, 6.
This moderate-sized anole characteristically inhabits the low bushes and bases of trees in the forest. Individuals were most readily observed on the buttresses of some of the gigantic mahogany and ceiba trees. When approached the lizards usually ran around the tree or ducked to the other side of the buttress; if the observer moved closer, they jumped to the ground and ran off. None was observed to ascend large trees. Some individuals were observed foraging on the forest floor; these took shelter on the bases of trees. One individual was sleeping on a palm frond at night. The adult males have a uniformly orange-red dewlap.
Anolis limifrons rodriguezi Bocourt
15 km. NW of Chinajá, 2; 20 km. NNW of Chinajá, 1.
In dry forests and more open situations than occur at Chinajá this little anole is abundant, but in the wet forests of southern El Petén, only three specimens were found. Two were on palm fronds about two meters above the ground; the other was on a low bush. I suspect that ecologically this species overlaps A. humilis uniformis and A. lemurinus bourgeaei, but too few observations are recorded to justify a definite statement at this time.
Anolis sericeus sericeus Hallowell
Chinajá, 2; Sayaxché, 1; Toocog, 1.
This small anole is common and widespread in the Atlantic lowlands of southern México and northern Central America; usually it inhabits sub-humid regions. Consequently, its presence in the wet forests of southern El Petén was unexpected. The specimens from Chinajá were sleeping on low bushes at night, whereas the others were found on bushes by day.
Basiliscus vittatus Wiegmann
Chinajá, 6; Río de la Pasión, 1; Río San Román, 1; Sayaxché, 3; Toocog, 1.
Individuals of this abundant species were most frequently seen in dense bushes along the margins of rivers or small streams. None was observed far from water. These lizards, like the anoles, are known locally as toloque.
Corythophanes cristatus (Merrem)
Chinajá, 3; 20 km. NNW of Chinajá, 1.
Three individuals were found on tree trunks; the fourth was on a thick vine about one meter above the ground. The two largest males have snout-vent lengths of 121 and 115 mm. and tail lengths of 265 and 243 mm. The largest female (KU 59603), obtained on June 28, has a snout-vent length of 125 mm. and a tail length of 247 mm. This individual contained eight ova varying in greatest diameter from 10.6 to 12.2 (average 11.1) mm. Also present are numerous ovarian eggs having diameters up to about 3.5 mm.
One of the large males displayed a defensive behavior prior to capture. When first observed the lizard was clinging to a tree trunk about one and one-half meters above the ground. When I approached, the lizard turned its flanks towards me; then it flattened the body laterally, extended the dewlap, opened its mouth, and made short rushing motions. When touched it bit viciously. On the ground these lizards have a rather awkward bipedal gait that is much slower than in Basiliscus vittatus.
In life an adult male (KU 55804) was reddish brown dorsally with dark chocolate brown markings; the venter was creamy white, and the iris was dark red. The natives call this lizard piende jente.
Iguana iguana rhinolopha Wiegmann
Río San Román, 2.
The iguana, as this lizard is called locally, seems to be uncommon in the forested areas of southern El Petén. Possibly this is due to the fact that the flesh of this lizard is relished as food by the natives. My two specimens were in large trees at the edge of the river.
Laemanctus deborrei Boulenger
Chinajá, 1; Toocog, 5.
On June 26 a female having a snout-vent length of 129 mm. and a tail length of 502 mm. was found on a bush in the forest. The lizard, when approached, faced the collector and opened its mouth. In life the dorsum was bright green; the lateral stripe was white, and the iris was yellowish brown. This specimen contained four ova having lengths of 13.4 to 14.2 (average 13.9) mm.
On June 30 at Toocog five white-shelled eggs were found in a rotting log. Measurements of the eggs are—length, 23.5 to 25.0 (average 24.2) mm.; width, 15.0 to 15.5 (average 15.4) mm. These eggs hatched on August 30. The five young had snout-vent lengths[236] of 43 to 45 (average 44) mm., and tail lengths of 137 to 140 (average 138) mm. In life the hatchlings had a dull dark green dorsum, pale bright green venter and stripes on head, and reddish brown iris. In preservative the hatchlings are creamy tan above with five or six square dark brown blotches middorsally.
The natives consider this lizard to be one of the anoles; consequently, it is known as toloque.
Lepidophyma flavimaculatum flavimaculatum Duméril
Chinajá, 8; 15 km. NW of Chinajá, 2.
Individuals were found beneath logs on the forest floor or moving about in the litter on the forest floor. One was observed crawling across a trail during a heavy rain. In some adults the tan dorsal spots are large and distinct; in others the spots are small and indistinct. Two juveniles, apparently recent hatchlings, were found on June 28 and July 5. These specimens have snout-vent lengths of 29 mm. and tail lengths of 38 and 41 mm.
Eumeces schwartzei Fischer
Chinajá, 1.
One specimen (KU 59551) was found on the forest floor at midday; it is an adult female having a snout-vent length of 125 mm. and a tail length of 210 mm. This specimen is larger than those recorded by Taylor (1936:99) and extends the known range of the species south of Ramate, approximately 125 kilometers south-south-westward to Chinajá.
Eumeces sumichrasti (Cope)
20 km. NNW of Chinajá, 1.
One adult male having a snout-vent length of 82 mm. was found beneath a palm frond on the forest floor. In life the dorsum was dull brown; the chin was cream; the belly was yellow, and the underside of the tail was orange. A juvenile having a black body, yellow dorsal stripes, and a bright blue tail was observed on the forest floor.
Scincella cherriei cherriei (Cope)
Chinajá, 2; 30 km. NNW of Chinajá, 1; Toocog, 1.
All individuals of this lizard were found in the leaf litter on the forest floor; many escaped capture. In life the tail is dull bluish gray. The number of dorsal scales varies from 59 to 61 (average[237] 60); thus, these specimens fall within the range of variation of S. cherriei cherriei, and thereby differ from S. cherriei stuarti to the west and S. cherriei ixbaac to the north.
Ameiva festiva edwardsi Bocourt
Chinajá, 16; 15 km. NW of Chinajá, 10; Sayaché, 4; Toocog, 1.
This abundant terrestrial lizard, locally called lagartijo, is found throughout the forest. A juvenile obtained on March 14 at Sayaxché has a snout-vent length of 42 mm. and a prominent umbilical scar. Other juveniles were observed at Chinajá in February and March, thereby indicating that the young probably hatch in the early part of the year. Juveniles have bright blue tails.
Celestus rozellae Smith
20 km. NNW of Chinajá, 2.
Two specimens were obtained from trees by workmen in February. These lizards have snout-vent lengths of 70 and 83 mm. and tail lengths of 133 and 135 mm. There are 21 and 23 lamellae beneath the fourth toe; each has 31 longitudinal rows of scales around the body.
Boa constrictor imperator Daudin
15 km. NW of Chinajá, 1; 20 km. NNW of Chinajá, 2; Toocog, 1.
All specimens were found on the forest floor. One individual was found in combat with a large Drymarchon corais melanurus. Apparently, the Drymarchon was attempting to devour the Boa, which had a total length of 1683 mm. Locally this snake is called masacuata; it is one of the few snakes believed by the local inhabitants to be non-poisonous.
Clelia clelia clelia Daudin
15 km. NW of Chinajá, 1; 20 km. NNW of Chinajá, 1.
One specimen is represented only by the head; the snake was killed on the forest floor by workmen. Another individual was found in a pool of water at the base of a limestone outcropping in the forest; this specimen (KU 58167) is a female having a body length of 2220 mm. and a total length of 2634 mm. This snake contained 22 ova averaging 56 × 23 mm. Both specimens were uniform shiny black above and cream-color below. The local name is sumbadora.
Coniophanes bipunctatus bipunctatus (Günther)
Chinajá, 1.
This snake was found on the forest floor by day; it is a male having 130 ventrals, an incomplete tail; cream-colored belly, and a pair of large brown spots on each ventral scute.
Coniophanes fissidens fissidens (Günther)
Toocog, 1.
This male specimen was found beneath a rock in a sink hole. It has 122 ventrals and 77 caudals. A narrow temporal stripe extends along the upper edge of the anterior temporal and the lower edge of the upper secondary temporal. The belly is ashy white with a pair of small black spots on each ventral.
Coniophanes imperialis clavatus (Peters)
Chinajá, 3.
All specimens were found on the forest floor by day. These small snakes are capable of rapid movement and quickly disappear in the litter on the ground. Two individuals evaded capture. The belly is creamy white anteriorly and vermillion red posteriorly.
Dryadophis melanolomus laevis (Fischer)
Chinajá, 3.
These snakes, locally known as sumbadora, were found on the forest floor; two others were seen, but escaped. The variation in coloration has been a source of confusion in this species in northern Central America (see Stuart, 1941:86). All of the present specimens are males: KU 55709 has 178 ventrals, 121 caudals, and a total length of 914 mm.; the dorsum is olive-tan with six darker cross-bars on the neck; the belly is creamy white. KU 58160 has 188 ventrals, 123 caudals, and a total length of 1365 mm.; the dorsum is uniform olive-brown, except that some dorsal scales at midbody have black anterior borders like D. melanolomus melanolomus has in the Yucatán Peninsula; the venter is pale yellow. KU 58158 has 179 ventrals, 122 caudals, and a total length of 723 mm.; the dorsum is rich chocolate brown with eight dark cross-bars on the neck; the belly is bright orange.
Stuart (1941a:87) stated that in life two distinct color phases were observed in specimens collected by him in Alta Verapaz, Guatemala. One had an olive-brown dorsum and the other, a[239] reddish orange dorsum. Stuart made no mention of variation in the color of the venter. Similar variation is known in D. melanolomus alternatus in Costa Rica, where some individuals have orange-red venters. This color phase has been recognized as a distinct species, Dryadophis sanguiventris, by Taylor (1954:722). Examination of 18 specimens from Costa Rica shows no differences in scutellation, nor geographic segregation of two populations. I am convinced that the red-bellied Dryadophis in Costa Rica, like those in Guatemala, represent a color phase of the subspecies inhabiting those areas and that Dryadophis sanguiventris Taylor is a synonym of Dryadophis melanolomus alternatus (Bocourt).
Drymarchon corais melanurus (Duméril, Bibron and Duméril)
15 km. NW of Chinajá, 1; Sayaxché, 1.
The specimen from Sayaxché was found at the edge of a clearing in the forest; that from 15 kilometers northwest of Chinajá was found on the forest floor coiled with a Boa constrictor imperator, which the Drymarchon apparently was trying to eat. The Drymarchon is a giant specimen having a total length of 2950 mm. (see Duellman, 1961:368). The Boa with which it was coiled has a total length of 1683 mm. I was attracted to the snakes by a loud thrashing noise. When I approached the writhing mass, the snakes separated, but I was able to see that the Drymarchon had its teeth firmly imbedded in the posterior part of the head of the Boa. From the Drymarchon I forced the regurgitation of a recently ingested Bothrops nummifer nummifer having a total length of 953 mm. These observations show that the snake-eating capabilities of Drymarchon can hardly be over-estimated.
In both Drymarchon the anterior one-half of the body is olive-tan, which changes to bluish black posteriorly. The local name is sumbadora.
Drymobius margaritiferus margaritiferus (Schlegel)
Chinajá, 3; Sayaxché, 1.
All individuals were obtained in clearings in the forest by day in the rainy season. Two individuals each contained a Similisca baudini and another contained a Bufo valliceps valliceps. Locally this snake is known by the appropriate name of ranera.
Imantodes cenchoa leucomelas Cope
Chinajá, 4.
With the exception of one that was found dead in camp, all[240] individuals were taken from low vegetation by day. The dorsum is creamy tan with 28 to 35 (average 32) chocolate brown blotches, and the venter is ashy white with small brown flecks. Three males have 238 to 248 (average 244) ventrals and 148 to 154 (average 151) caudals; one female has 239 ventrals and 142 caudals. The largest specimen, a male, has a body length of 660 mm. and a total length of 943 mm.
Lampropeltis doliata polyzona Cope
Chinajá, 1.
One female (KU 57156) having 230 ventrals and 54 caudals was found on the forest floor by day. This individual has a black snout with a white bar across the nasals and prefrontals, a white spot in the middle of the frontal, and a white band across the temporals and parietals that is bordered posteriorly by a black band. There are 28 white and 28 red rings on the body. The tips of the red scales are darkened. The black rings between the white and red rings are not so expanded as to interrupt the white rings dorsally as in L. doliata abnorma as identified by Stuart (1948:70). Locally this snake, like all red, black, and white or yellow banded snakes, is called coral or coralillo.
Leptodeira frenata malleisi Dunn and Stuart
Toocog, 1.
This specimen, a male having 173 ventrals and 69 caudals, was found beneath the bark on a log in the forest. In life the dorsum was pinkish tan with 36 chocolate brown blotches on the body; the venter was rosy pink.
Leptodeira septentrionalis polysticta Günther
Chinajá, 3; Toocog, 11.
If numbers of specimens are indicative of abundance, this is the most common snake in southern El Petén. All were found at night in the rainy season. At a pond in the forest at Toocog these snakes were observed on low vegetation, on the ground, and in the water. Evidently they congregate at breeding choruses of frogs. One Leptodeira contained a Smilisca baudini and another contained eggs of Phyllomedusa callidryas taylori. The natives call this snake nahuyaca.
Leptophis ahaetulla praestans (Cope)
13 km. NNW of Chinajá, 1; 20 km. NNW of Chinajá, 1.
Both specimens were obtained from trees when they were felled. One individual (KU 55716) has a body length of 1345 mm. and a total length of 2035 mm. In life the entire snake was uniform bright green; the eye was yellow. In preservative the dorsum is dark blue, and the venter is green.
Leptophis mexicanus mexicanus Duméril, Bibron and Duméril
Chinajá, 1; 15 km. NW of Chinajá, 1; Sayaxché, 4.
All specimens came from low trees in the forest. The largest specimen is a male having a body length of 724 mm. and a total length of 1236 mm. In life the middorsum was a golden tan; the top of the head was a vivid green. One individual had ingested a Smilisca baudini. The local name is bejuquillo.
Ninia sebae sebae (Duméril, Bibron and Duméril)
Toocog, 1.
This specimen, a male having 144 ventrals and 55 caudals, was found beneath bark on a log in the forest. There is a black band five scales in length on the nape followed posteriorly by a red band six scales in length and then by a complete black band one and one-half scales in length. The rest of the body is dull red with 16 incomplete black bands one to one and one-half scales in length on the anterior two-thirds of the body.
Oxybelis aeneus aeneus (Wagler)
Chinajá, 1; 20 km. NNW of Chinajá, 1.
One individual was found in a low tree; the other was in a bush. Both specimens are males; the largest has a body length of 754 mm. and a total length of 1286 mm. Bogert and Oliver (1945:388) distinguished O. aeneus aeneus in Central and South America from O. aeneus auratus in México in that the diameter of the eye is more than the length of the internasal, whereas in O. aeneus auratus the diameter of the eye is less than the length of the internasal. Stuart (1958:27) stated that on the basis of this character three specimens from Tikal in northeastern El Petén definitely were O. aeneus aeneus. Of the present specimens from southern El Petén, one has an internasal:eye ratio of 1.08; the other has a ratio of 0.87. A careful review of these snakes is needed to verify the validity of the characters used to separate the subspecies[242] and to determine areas of intergradation. The local name for the vine-snake is bejuquillo.
Pliocercus euryzonus aequalis Salvin
Chinajá, 1; Río San Román, 1.
These specimens are tentatively referred to P. euryzonus. KU 57160 is a female having 130 ventrals, 87 caudals, and 23 black rings on the body; KU 58150 is a juvenile having 128 ventrals, 79 caudals, and 27 black rings on the body. In both specimens the tip of the snout is yellow; a broad yellow band on the parietals and temporals is bordered posteriorly by a black band on the nape. The black rings on the body are not bordered by yellow, but black rings on the tail have yellow borders ventrally. In the red interspaces between the black rings, black flecks and spots, especially posteriorly, tend to form secondary black rings (Fig. 6a). According to Stuart (1948:71), P. euryzonus aequalis has 25 to 27 black rings on the body, whereas P. elapoides salvini, which also occurs in El Petén, has 15 to 23 black rings.
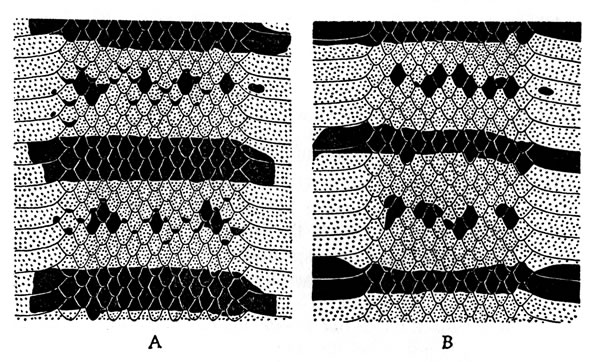 Fig. 6. Dorsal color patterns of Pliocercus euryzonus aequalis (A) and Micrurus affinis apiatus (B).
Fig. 6. Dorsal color patterns of Pliocercus euryzonus aequalis (A) and Micrurus affinis apiatus (B).
The specimen from the Río San Román contained a partly digested Bolitoglossa moreleti mulleri. Locally Piocercus is called coral or coralillo.
Pseustes poecilonotus poecilonotus (Günther)
Chinajá, 3; 20 km. NNW of Chinajá, 1.
Two juveniles were on the forest floor; one juvenile and an adult were on low bushes. The juveniles have a tan dorsum with reddish brown blotches; the belly is gray, and the iris is cream-color above and brown below. The one adult is olive-brown above and creamy white below on the anterior three-fourths of the body; posteriorly it is black above and below. There are no paravertebral dark stripes nor pale spots on the dorsal scales.
Two specimens (one juvenile and the adult) when encountered compressed the anterior part of the body laterally and struck repeatedly. Locally the adults are called sumbadora.
Sibon dimidiata dimidiata (Günther)
20 km. NNW of Chinajá, 2.
Both snakes were obtained from trees when they were felled. In life the dorsum was pinkish orange with dark chocolate brown blotches narrowly edged with black.
Sibon nebulata nebulata (Linnaeus)
20 km. NW of Chinajá, 1.
This specimen, a male having a body length of 544 mm. and a tail length of 198 mm., was found in a felled tree. In life the belly was pink and black; the dorsal black blotches were narrowly outlined with pink.
Spilotes pullatus mexicanus (Laurenti)
Chinajá, 3; 20 km. NNW of Chinajá, 1; Sayaxché, 1.
This large snake, locally called mica, seems to be equally at home on the ground and in low trees and bushes. It is fast moving for a large snake; two individuals escaped capture. The natives said that this snake eats other snakes, but examination of stomachs revealed no supporting evidence.
Stenorrhina degenhardti (Berthold)
Chinajá, 1.
This specimen, a female having 158 ventrals, 37 caudals, and a total length of 489 mm., was found on the forest floor. On the olive-brown dorsum are 27 irregular, narrow, dark brown, transverse bands. The head is uniform olive-brown; the chin and labials are[244] cream-color. The venter is cream-color with a row of brown spots forming a midventral stripe. A large spider was found in the stomach.
I have refrained from assigning a subspecific name to this snake. Cursory examination of specimens from throughout México and Central America reveals a bewildering array of variation in coloration that suggests that the subspecies mexicanus is not recognizable, or that two species occur sympatrically in parts of southern México and northern Central America.
Tretanorhinus nigroluteus lateralis Bocourt
Chinajá, 1.
A single male having 136 ventrals, 75 caudals, and a total length of 407 mm. was found by a stream in camp. The dorsum is pale grayish tan with 34 pairs of small chocolate brown spots, some of the anterior ones of which are connected across the back. A cream-colored lateral stripe is on the third and fourth dorsal scale-rows anteriorly and the second and third rows posteriorly. The lower dorsal scale rows are black. The venter is dark grayish brown with cream-colored flecks anteriorly and creamy gray posteriorly where the dark color is restricted to the midventral region and the lateral edges of ventrals and first dorsal scale-row.
Xenodon rabdocephalus mexicanus Smith
Chinajá, 1; 20 km. NNW of Chinajá, 1.
Both individuals were found on the forest floor. An adult male having a total length of 420 mm. has a cream-colored venter with brown flecks. A juvenile having a total length of 172 mm. has a creamy white belly with black crossbands.
At the suggestion of L. C. Stuart, I am following Schmidt (1941:501) in placing X. mexicanus as a subspecies of X. rabdocephalus.
Micrurus affinis apiatus (Jan)
20 km. NNW of Chinajá, 2; Sayaxché, 1.
All specimens were found beneath litter on the forest floor. All are males having 202 to 211 (average 205) ventrals, 53 to 56 (54.6) caudals, and 34 to 48 (41) primary black rings on the body. There are no yellow rings, and black spots in the red interspaces tend to form secondary black rings (Fig. 6b), the same as in Pliocercus euryzonus aequalis. The local name is coral or coralillo.
Bothrops atrox asper (Garman)
15 km. NW of Chinajá, 1; Sayaxché, 1.
Although we found only two specimens, natives and workmen at the camp at Chinajá stated that the barba amarilla, as this snake is known locally, had been abundant when the camp had been established less than two years before our visit.
Bothrops nasutus Bocourt
12 km. NW of Chinajá, 1.
This specimen, a male having a total length of 415 mm., was found on the forest floor. The dorsum is brown with dark brown blotches separated middorsally by a narrow orange-tan stripe extending from the nape to the base of the tail. The belly is grayish tan with white flecks on the lateral edges of the ventrals. The local name is nahuyaca.
Bothrops nummifer nummifer (Rüppell)
15 km. NW of Chinajá, 2; Sayaxché, 1.
Two individuals were found on the forest floor, and one adult, having a total length of 953 mm., was removed from the stomach of a large Drymarchon corais melanurus. There is considerable variation in color and pattern. A juvenile (KU 58104), having a total length of 332 mm., has a tan dorsum with 19 interconnected dark brown, diamond-shaped, middorsal blotches, the lateral extensions of which are black; the belly is a cream-color with brown squares. An adult female (KU 55706), having a total length of 779 mm., has a dorsal coloration like the preceding specimen, except that the lateral extensions of the dorsal blotches are brown; the belly is a uniform cream-color. A second adult female (KU 55707), having a total length of 953 mm., has a brown dorsum with 21 interconnected black, diamond-shaped, middorsal blotches, the lateral extensions of which are black; the belly is a cream-color with black squares.
The local name for this species is braza de piedra.
Bothrops schlegeli schlegeli (Berthold)
Paso Subín, 1.
This specimen was taken from the thatched roof of a house at the edge of the forest and contained the remains of a small mammal. The local name is nahuyaca.
HYPOTHETICAL LIST OF SPECIES
Listed below are thirteen species that have not been found in southern El Petén but that probably occur there.
Dermophis mexicanus mexicanus (Duméril and Bibron).—Natives at Chinajá know caecilians, which they call dos cabezas. This species has been taken in Tabasco and northern Chiapas. Its occurrence in southern El Petén is expected. Less likely, the caecilian known to the natives at Chinajá is Gymnopis oligozona, which is known from Finca Volcán on the southern slopes of the valley of the Río Cahabón in Alta Verapaz.
Gastrophryne elegans (Boulenger).—This small fossorial frog is known from Piedras Negras (Taylor and Smith, 1945:604), 12 miles east of Yaxha (Stuart, 1934:7), and Tikal (Stuart, 1958:18), all in northern and central El Petén. Two specimens in the collection of the University of Kansas are from 28 kilometers northeast of Campur, Alta Verapaz. Probably the species ranges throughout the forested lowlands of northern Alta Verapaz and El Petén.
Mabuya brachypoda Taylor.—The absence of this widespread lizard in our collections cannot be explained. Probably it occurs in southern El Petén, for it is known in northern and central El Petén and in Alta Verapaz.
Dendrophidion vinitor Smith.—This snake is known from Piedras Negras, El Petén and from various localities in Alta Verapaz; it is an inhabitant of humid forest and should occur in southern El Petén.
Elaphe triaspis mutabilis (Cope).—The subspecies E. triaspis mutabilis is known from Alta Verapaz and E. triaspis triaspis from the Yucatán Peninsula, British Honduras, and Uaxactún in northern El Petén. Because of the much higher degree of resemblance between the faunas of southern El Petén and Alta Verapaz as compared with southern El Petén and Yucatán, E. triaspis mutabilis would be expected to occur in southern El Petén.
Ninia diademata nietoi Burger and Werler.—This snake is known from Tikal and from Alta Verapaz; it is a small cryptophile that probably occurs in southern El Petén.
Oxyrhophus petola aequifasciatus Werner.—This snake, which probably is conspecific with Oxyrhophus baileyi in southern Veracruz, México, is known from Tikal, British Honduras, and Alta Verapaz; it is expected in southern El Petén.
Pliocercus elapoides salvini Müller.—This species is widespread in the Atlantic lowlands of southern México and northern Central America; the subspecies P. elapoides salvini occurs in Alta Verapaz and probably in southern El Petén.
Rhadinaea decorata decorata (Günther).—This is another small cryptophile that is widespread on the Atlantic lowlands from México to Panamá; it definitely is expected at places like Chinajá in southern El Petén.
Scaphiodontophis annulatus (Duméril and Bibron).—Three subspecies of Scaphiodontophis annulatus are recognized in northern Central America: S. annulatus annulatus from Alta Verapaz, S. annulatus hondurensis from northern Honduras, and S. annulatus carpicinctus from Piedras Negras and[247] Tikal in El Petén and from British Honduras. This rare and highly variable species probably occurs in southern El Petén.
Tantilla schistosa schistosa (Bocourt).—This widespread species in Central America is known from several localities in Alta Verapaz and almost certainly occurs in southern El Petén.
Tropidodipsas sartori sartori Cope.—This fossorial species has been collected in northern El Petén and in Alta Verapaz. The natives at Chinajá described to me a coral having orange rings on a black body that likely was this species.
Micrurus elegans veraepacis Schmidt.—This species has been collected at various localities in Alta Verapaz and in Chiapas, inhabits areas like those in southern El Petén, and probably occurs there.
SUMMARY
A study of the amphibians and reptiles in the rainforests of southern El Petén, Guatemala, reveals the presence of 78 species; an additional 13 species probably occur there. In this tropical area having a high amount of rainfall most of the species of amphibians and reptiles have extensive ranges in the wet forests on the Atlantic lowlands of southern México and northern Central America; some species that more frequently are found in sub-humid forests also occur.
Ecologically the fauna is divided into five major habitats—aquatic, aquatic margin, fossorial, terrestrial, and arboreal. Forty-two per cent of the 78 species are wholly or partly arboreal. The fauna is most closely related to that in Alta Verapaz, Guatemala, but includes many species that occur in the Tikal-Uaxactún area in northeastern Guatemala.
Eleutherodactylus rostralis (Werner) and E. rhodopis (Cope) are redefined and their relationships are suggested. The color phases of Dryadophis melanolomus laevis and D. m. alternatus are discussed; Dryadophis sanguiventris Taylor is synonymized with Dryadophis melanolomus alternatus (Bocourt).
The breeding habits, eggs, and tadpoles of the hylid frogs Hyla ebraccata and Phyllomedusa callidryas taylori are described, as are the eggs and juveniles of Laemanctus deborrei.
LITERATURE CITED
Baylor, E. R. and Stuart, L. C.
1961. A new race of Bufo valliceps from Guatemala. Proc. Biol. Soc. Washington, 74:195-202, August 11.
Bogert, C. M. and Oliver, J. A.
1945. A preliminary analysis of the herpetofauna of Sonora. Bull. Amer. Mus. Nat. Hist., 83:297-426, March 30.
Brocchi, P.
1881-1883 Étude des batraciens de l'Amerique Centrale. Mission scientifique au Mexique. Paris, Imprimerie Nationale, 3 (2):1-122, pls. 1-21.
Duellman, W. E.
1958. A review of the frogs of the genus Syrrhophus in western Mexico. Occas. Papers Mus. Zool. Univ. Michigan, 594:1-15, pls. 1-3, June 6.
1960. A distributional study of the amphibians of the Isthmus of Tehuantepec, México. Univ. Kansas Publ. Mus. Nat. Hist., 13:21-72, August 16.
1961. A record size for Drymarchon corais melanurus. Copeia, 1960 (4):367-368, January.
Dunn, E. R. and Emlen, J. T.
1932. Reptiles and amphibians from Honduras. Proc. Acad. Nat. Sci. Philadelphia, 84:21-32, March 22.
Firschein, I. L. and Smith, H. M.
1957. A high-crested race of toad (Bufo valliceps) and other noteworthy reptiles and amphibians from southern Mexico. Herpetologica, 13:219-222, October 31.
Lundell, C. L.
1937. The vegetation of Petén. Carnegie Institute Washington Publ. 178:1-244, pls. 1-39. June 16.
Neill, W. T. and Allen, R.
1959. Studies on the amphibians and reptiles of British Honduras. Publ. Ross Allen's Reptile Inst., 2:1-76, November 10.
Sapper, K.
1932. Klimakunde von Mittelamerika. In Handbuch Klimakunde, 2:1-74, Taf. 1-13.
Schmidt, K. P.
1936. Guatemalan salamanders of the genus Oedipus. Zool. Ser. Field Mus. Nat. Hist., 20:135-166, October 31.
1941. The amphibians and reptiles of British Honduras. Zool. Ser. Field Mus. Nat. Hist, 22:475-510, December 30.
1946. Turtles collected by the Smithsonian Biological Survey of the Panamá Canal Zone. Smithsonian Misc. Coll., 106 (8):1-9, pl. 1, August 1.
Simpson, G. G.
1960. Notes on the measurement of faunal resemblance. Amer. Jour. Sci., 258-A:300-311.
Smith, H. M. and Taylor, E. H.
1945. An annotated checklist and key to the snakes of Mexico. Bull. U. S. Natl. Mus., 187: iv + 239 pp., October 5.
1948. An annotated checklist and key to the amphibia of Mexico. Bull. U. S. Natl. Mus., 194: iv + 118 pp., June 17.
1950. An annotated checklist and key to the reptiles of Mexico exclusive of the snakes. Bull. U. S. Natl. Mus., 199: v + 253 pp., October 26.
Stuart, L. C.
1934. A contribution to a knowledge of the herpetological fauna of El Peten, Guatemala. Occas. Papers Mus. Zool. Univ. Michigan, 292:1-18, June 29.
1935. A contribution to a knowledge of the herpetology of a portion of the savanna region of central Petén, Guatemala. Misc. Publ. Mus. Zool. Univ. Michigan, 29:1-56, pls. 1-4, October 1.
1937. Some further notes on the amphibians and reptiles of the Peten forest of northern Guatemala. Copeia, 1937 (1):67-70, April 10.
1941a. Studies of Neotropical Colubrinae VIII. A revision of the genus Dryadophis Stuart, 1939. Misc. Publ. Mus. Zool. Univ. Michigan, 49:1-105, pls. 1-4, March 19.
1941b. Two new species of Eleutherodactylus from Guatemala. Proc. Biol. Soc. Washington, 54:197-200, December 8.
1943. Taxonomic and geographic comments on Guatemalan salamanders of the genus Oedipus. Misc. Publ. Mus. Zool. Univ. Michigan, 56:1-33, pls. 1-2, January 30.
1948. The amphibians and reptiles of Alta Verapaz, Guatemala. Misc. Publ. Mus. Zool. Univ. Michigan, 69:1-109, June 12.
1950. A geographic study of the herpetofauna of Alta Verapaz, Guatemala. Contr. Lab. Vert. Biol. Univ. Michigan, 45:1-77, pls. 1-9, May.
1958. A study of the herpetofauna of the Uaxactun-Tikal area of northern El Peten, Guatemala. Contr. Lab. Vert. Biol. Univ. Michigan, 75:1-30, June.
Taylor, E. H.
1936. A taxonomic study of the cosmopolitan scincoid lizards of the genus Eumeces. Univ. Kansas Sci. Bull., 23:1-643, August 15.
1954. Further studies on the serpents of Costa Rica. Univ. Kansas Sci. Bull., 36:673-801, July 15.
Taylor, E. H. and Smith, H. M.
1945. Summary of collections of amphibians made in Mexico under the Walter Rathbone Bacon Traveling Scholarship. Proc. U. S. Natl. Mus., 95:521-613, June 30.
Transmitted November 29, 1962.
29-5935
UNIVERSITY OF KANSAS PUBLICATIONS MUSEUM OF NATURAL HISTORY
Institutional libraries interested in publications exchange may obtain this series by addressing the Exchange Librarian, University of Kansas Library, Lawrence, Kansas. Copies for individuals, persons working in a particular field of study, may be obtained by addressing instead the Museum of Natural History, University of Kansas, Lawrence, Kansas. There is no provision for sale of this series by the University Library, which meets institutional requests, or by the Museum of Natural History, which meets the requests of individuals. However, when individuals request copies from the Museum, 25 cents should be included, for each separate number that is 100 pages or more in length, for the purpose of defraying the costs of wrapping and mailing.
* An asterisk designates those numbers of which the Museum's supply (not the Library's supply) is exhausted. Numbers published to date, in this series, are as follows:
Vol. 1. Nos. 1-26 and index. Pp. 1-638, 1946-1950.
*Vol. 2. (Complete) Mammals of Washington. By Walter W. Dalquest. Pp. 1-444, 140
figures in text. April 9, 1948.
Vol. 3. *1. The avifauna of Micronesia, its origin, evolution, and distribution. By Rollin
H. Baker. Pp. 1-359, 16 figures in text. June 19, 1951.
*2. A quantitative study of the nocturnal migration Of birds. By George H.
Lowery, Jr. Pp. 361-472, 47 figures in text. June 29, 1951.
3. Phylogeny of the waxwings and allied birds. By M. Dale Arvey. Pp. 473-530,
49 figures in text, 13 tables. October 10, 1951.
*4. Birds from the state of Veracruz, Mexico. By George H. Lowery, Jr., and
Walter W. Dalquest. Pp. 531-649, 7 figures in text, 2 tables. October 10,
1951.
Index. Pp. 651-681.
*Vol. 4. (Complete) American weasels. By E. Raymond Hall. Pp. 1-466, 41 plates, 31
figures in text. December 27, 1951.
Vol. 5. Nos. 1-37 and index. Pp. 1-676, 1951-1953.
*Vol. 6. (Complete) Mammals of Utah, taxonomy and distribution. By Stephen D.
Durrant. Pp. 1-549, 91 figures in text, 30 tables. August 10, 1952.
Vol. 7. Nos. 1-15 and index. Pp. 1-651, 1952-1955.
Vol. 8. Nos. 1-10 and index. Pp. 1-675. 1954-1956.
Vol. 9. *1. Speciation of the wandering shrew. By James S. Findley. Pp. 1-68, 18
figures in text. December 10, 1955.
2. Additional records and extension of ranges of mammals from Utah. By
Stephen D. Durrant, M. Raymond Lee, and Richard M. Hansen. Pp. 69-80.
December 10, 1955.
3. A new long-eared myotis (Myotis evotis) from northeastern Mexico. By Rollin
H. Baker and Howard J. Stains. Pp. 81-84. December 10, 1955.
4. Subspeciation in the meadow mouse, Microtus pennsylvanicus, in Wyoming.
By Sydney Anderson. Pp. 85-104, 2 figures in text. May 10, 1956.
5. The condylarth genus Ellipsodon. By Robert W. Wilson. Pp. 105-116, 6
figures in text. May 19, 1956.
6. Additional remains of the multituberculate genus Eucosmodon. By Robert
W. Wilson. Pp. 117-123, 10 figures in text. May 19, 1956.
7. Mammals of Coahuila, Mexico. By Rollin H. Baker. Pp. 125-335, 75 figures
in text. June 15, 1956.
8. Comments on the taxonomic status of Apodemus peninsulae, with description
of a new subspecies from North China. By J. Knox Jones, Jr. Pp. 337-346,
1 figure in text, 1 table. August 15, 1956.
9. Extensions of known ranges of Mexican bats. By Sydney Anderson. Pp.
347-351. August 15, 1956.
10. A new bat (Genus Leptonycteris) from Coahuila. By Howard J. Stains.
Pp. 353-356. January 21, 1957.
11. A new species of pocket gopher (Genus Pappogeomys) from Jalisco, Mexico.
By Robert J. Russell. Pp. 357-361. January 21, 1957.
12. Geographic variation in the pocket gopher, Thomomys bottae, in Colorado.
By Phillip M. Youngman. Pp. 363-387, 7 figures in text. February 21, 1958.
13. New bog lemming (genus Synaptomys) from Nebraska. By J. Knox Jones,
Jr. Pp. 385-388. May 12, 1958.
14. Pleistocene bats from San Josecito Cave, Nuevo León, México. By J. Knox
Jones, Jr. Pp. 389-396. December 19, 1958.
15. New subspecies of the rodent Baiomys from Central America. By Robert
L. Packard. Pp. 397-404. December 19, 1958.
16. Mammals of the Grand Mesa, Colorado. By Sydney Anderson. Pp. 405-414,
1 figure in text. May 20, 1959.
17. Distribution, variation, and relationships of the montane vole, Microtus montanus.
By Sydney Anderson. Pp. 415-511, 12 figures in text, 2 tables.
August 1, 1959.
18. Conspecificity of two pocket mice, Perognathus goldmani and P. artus. By
E. Raymond Hall and Marilyn Bailey Ogilvie. Pp. 513-518, 1 map. January
14, 1960.
19. Records of harvest mice, Reithrodontomys, from Central America, with description
of a new subspecies from Nicaragua. By Sydney Anderson and
J. Knox Jones, Jr. Pp. 519-529. January 14, 1960.
20. Small carnivores from San Josecito Cave (Pleistocene), Nuevo León, México.
By E. Raymond Hall. Pp. 531-538, 1 figure in text. January 14, 1960.
21. Pleistocene pocket gophers from San Josecito Cave, Nuevo León, México.
By Robert J. Russell. Pp. 539-548, 1 figure in text. January 14, 1960.
22. Review of the insectivores of Korea. By J. Knox Jones, Jr., and David H.
Johnson. Pp. 549-578. February 23, 1960.
23. Speciation and evolution of the pygmy mice, genus Baiomys. By Robert L.
Packard. Pp. 579-670, 4 plates, 12 figures in text. June 16, 1960.
Index. Pp. 671-690
Vol. 10. 1. Studies of birds killed in nocturnal migration. By Harrison B. Tordoff and
Robert M. Mengel. Pp. 1-44, 6 figures in text, 2 tables. September 12, 1956.
2. Comparative breeding behavior of Ammospiza caudacuta and A. maritima.
By Glen E. Woolfenden. Pp. 45-75, 6 plates, 1 figure. December 20, 1956.
3. The forest habitat of the University of Kansas Natural History Reservation.
By Henry S. Fitch and Ronald R. McGregor. Pp. 77-127, 2 plates, 7 figures
in text, 4 tables. December 31, 1956.
4. Aspects of reproduction and development in the prairie vole (Microtus ochrogaster).
By Henry S. Fitch. Pp. 129-161, 8 figures in text, 4 tables. December
19, 1957.
5. Birds found on the Arctic slope of northern Alaska. By James W. Bee.
Pp. 163-211, plates 9-10, 1 figure in text. March 12, 1958.
*6. The wood rats of Colorado: distribution and ecology. By Robert B. Finley,
Jr. Pp. 213-552, 34 plates, 8 figures in text, 35 tables. November 7, 1958.
7. Home ranges and movements of the eastern cottontail in Kansas. By Donald
W. Janes. Pp. 553-572, 4 plates, 3 figures in text. May 4, 1959.
8. Natural history of the salamander, Aneides hardyi. By Richard F. Johnston
and Gerhard A. Schad. Pp. 573-585. October 8, 1959.
9. A new subspecies of lizard, Cnemidophorus sacki, from Michoacán, México.
By William E. Duellman. Pp. 587-598, 2 figures in text. May 2, 1960.
10. A taxonomic study of the middle-American snake, Pituophis deppei. By
William E. Duellman. Pp. 599-610, 1 plate, 1 figure in text. May 2, 1960.
Index. Pp. 611-626.
Vol. 11. Nos. 1-10 and index. Pp. 1-703, 1958-1960.
Vol. 12. 1. Functional morphology of three bats: Sumops, Myotis, Macrotus. By Terry
A. Vaughan. Pp. 1-153, 4 plates, 24 figures in text. July 8, 1959.
*2. The ancestry of modern Amphibia: a review of the evidence. By Theodore
H. Eaton, Jr. Pp. 155-180, 10 figures in text. July 10, 1959.
3. The baculum in microtine rodents. By Sydney Anderson. Pp. 181-216, 49
figures in text. February 19, 1960.
*4. A new order of fishlike Amphibia from the Pennsylvanian of Kansas. By
Theodore H. Eaton, Jr., and Peggy Lou Stewart. Pp. 217-240, 12 figures in
text. May 2, 1960.
5. Natural history of the bell vireo. By Jon C. Barlow. Pp. 241-296, 6 figures
in text. March 7, 1962.
6. Two new pelycosaurs from the lower Permian of Oklahoma. By Richard C.
Fox. Pp. 297-307, 6 figures in text. May 21, 1962.
7. Vertebrates from the barrier island of Tamaulipas, México. By Robert K.
Selander, Richard F. Johnston, B. J. Wilks, and Gerald G. Raun. Pp. 309-345,
pls. 5-8. June 18, 1962.
8. Teeth of Edestid sharks. By Theodore H. Eaton, Jr. Pp. 347-362, 10 figures
in text. October 1, 1962.
More numbers will appear in volume 12.
Vol. 13. 1. Five natural hybrid combinations in minnows (Cyprinidae). By Frank B.
Cross and W. L. Minckley. Pp. 1-18. June 1, 1960.
2. A distributional study of the amphibians of the Isthmus of Tehuantepec,
México. By William E. Duellman. Pp. 19-72, pls. 1-8, 3 figures in text.
August 16, 1960.
3. A new subspecies of the slider turtle (Pseudemys scripta) from Coahuila,
México. By John M. Legler. Pp. 73-84, pls. 9-12, 3 figures in text. August
16, 1960.
4. Autecology of the copperhead. By Henry S. Fitch. Pp. 85-288, pls. 13-20,
26 figures in text. November 30, 1960.
5. Occurrence of the garter snake, Thamnophis sirtalis, in the Great Plains and
Rocky Mountains. By Henry S. Fitch and T. Paul Maslin. Pp. 289-308,
4 figures in text. February 10, 1961.
6. Fishes of the Wakarusa river in Kansas. By James E. Deacon and Artie L.
Metcalf. Pp. 309-322, 1 figure in text. February 10, 1961.
7. Geographic variation in the North American cyprinid fish, Hybopsis gracilis.
By Leonard J. Olund and Frank B. Cross. Pp. 323-348, pls. 21-24, 2 figures
in text. February 10, 1961.
8. Descriptions of two species of frogs, genus Ptychohyla; studies of American
hylid frogs, V. By William E. Duellman. Pp. 349-357, pl. 25, 2 figures
in text. April 27, 1961.
9. Fish populations, following a drought, in the Neosho and Marais des Cygnes
rivers of Kansas. By James Everett Deacon. Pp. 359-427, pls. 26-30, 3 figs.
August 11, 1961.
10. Recent soft-shelled turtles of North America (family Trionychidae). By
Robert G. Webb. Pp. 429-611, pls. 31-54, 24 figures in text. February
16, 1962.
Index. Pp. 613-624.
Vol. 14. 1. Neotropical bats from western México. By Sydney Anderson. Pp. 1-8.
October 24, 1960.
2. Geographic variation in the harvest mouse. Reithrodontomys megalotis, on
the central Great Plains and in adjacent regions. By J. Knox Jones, Jr.,
and B. Mursaloglu. Pp. 9-27, 1 figure in text. July 24, 1961.
3. Mammals of Mesa Verde National Park, Colorado. By Sydney Anderson.
Pp. 29-67, pls. 1 and 2, 3 figures in text. July 24, 1961.
4. A new subspecies of the black myotis (bat) from eastern Mexico. By E.
Raymond Hall and Ticul Alvarez. Pp. 69-72, 1 figure in text. December
29, 1961.
5. North American yellow bats, "Dasypterus," and a list of the named kinds
of the genus Lasiurus Gray. By E. Raymond Hall and J. Knox Jones, Jr.
Pp. 73-98, 4 figures in text. December 29, 1961.
6. Natural history of the brush mouse (Peromyscus boylii) in Kansas with
description of a new subspecies. By Charles A. Long. Pp. 99-111, 1 figure
in text. December 29, 1961.
7. Taxonomic status of some mice of the Peromyscus boylii group in eastern
Mexico, with description of a new subspecies. By Ticul Alvarez. Pp. 113-120,
1 figure in text. December 29, 1961.
8. A new subspecies of ground squirrel (Spermophilus spilosoma) from Tamaulipas,
Mexico. By Ticul Alvarez. Pp. 121-124. March 7, 1962.
9. Taxonomic status of the free-tailed bat, Tadarida yucatanica Miller. By J.
Knox Jones, Jr., and Ticul Alvarez. Pp. 125-133, 1 figure in text. March 7,
1962.
10. A new doglike carnivore, genus Cynaretus, from the Clarendonian Pliocene,
of Texas. By E. Raymond Hall and Walter W. Dalquest. Pp. 135-138,
2 figures in text. April 30, 1962.
11. A new subspecies of wood rat (Neotoma) from northeastern Mexico. By
Ticul Alvarez. Pp. 139-143. April 30, 1962.
12. Noteworthy mammals from Sinaloa, Mexico. By J. Knox Jones, Jr., Ticul
Alvarez, and M. Raymond Lee. Pp. 145-159, 1 figure in text. May 18,
1962.
13. A new bat (Myotis) from Mexico. By E. Raymond Hall. Pp. 161-164,
1 figure in text. May 21, 1962.
14. The mammals of Veracruz. By E. Raymond Hall and Walter W. Dalquest.
Pp. 165-362, 2 figures. May 20, 1963.
15. The recent mammals of Tamaulipas, México. By Ticul Alvarez. Pp. 363-473,
5 figures in text. May 20, 1963.
More numbers will appear in volume 14.
Vol. 15. 1. The amphibians and reptiles of Michoacán, México. By William E. Duellman.
Pp. 1-148, pls. 1-6, 11 figures in text. December 20, 1961.
2. Some reptiles and amphibians from Korea. By Robert G. Webb, J. Knox
Jones, Jr., and George W. Byers. Pp. 149-173. January 31, 1962.
3. A new species of frog (Genus Tomodactylus) from western México. By
Robert G. Webb, Pp. 175-181, 1 figure in text. March 7, 1962.
4. Type specimens of amphibians and reptiles in the Museum of Natural History,
the University of Kansas. By William E. Duellman and Barbara Berg.
Pp. 183-204. October 26, 1962.
5. Amphibians and Reptiles of the Rainforests of Southern El Petén, Guatemala.
By William E. Duellman. Pp. 205-249, pls. 7-10, 6 figures in text. October
4, 1963.
More numbers will appear in volume 15.

