A DNA polymerase is an enzyme that catalyzes the polymerization of deoxyribonucleotides into a DNA strand. DNA polymerases are best-known for their role in DNA replication, in which the polymerase "reads" an intact DNA strand as a template and uses it to synthesize the new strand. This process copies a piece of DNA. The newly-polymerized molecule is complementary to the template strand and identical to the template's original partner strand. DNA polymerases use a magnesium ion for catalytic activity.
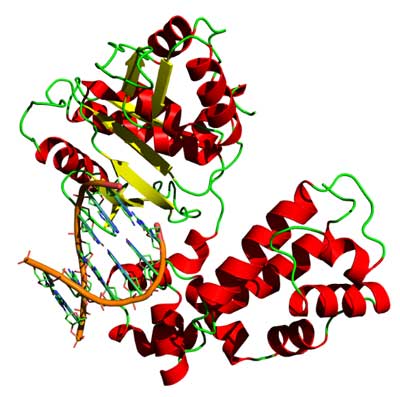
3D structure of the DNA-binding helix-turn-helix motifs in human DNA polymerase beta (*)
DNA polymerase can add free nucleotides to only the 3' end of the newly-forming strand. This results in elongation of the new strand in a 5'-3' direction. No known DNA polymerase is able to begin a new chain (de novo). DNA polymerase can add a nucleotide onto only a preexisting 3'-OH group, and, therefore, needs a primer at which it can add the first nucleotide. Primers consist of RNA and DNA bases with the first two bases always being RNA, and are synthesized by another enzyme called primase. An enzyme known as a helicase is required to unwind DNA from a double-strand structure to a single-strand structure to facilitate replication of each strand consistent with the semiconservative model of DNA replication. Error correction is a property of some, but not all, DNA polymerases. This process corrects mistakes in newly-synthesized DNA. When an incorrect base pair is recognized, DNA polymerase reverses its direction by one base pair of DNA. The 3'-5' exonuclease activity of the enzyme allows the incorrect base pair to be excised (this activity is known as proofreading). Following base excision, the polymerase can re-insert the correct base and replication can continue. Various DNA polymerases are extensively used in molecular biology experiments. Variation across species DNA polymerases have highly-conserved structure, which means that their overall catalytic subunits vary, on a whole, very little from species to species. Conserved structures usually indicate important, irreplicable functions of the cell, the maintenance of which provides evolutionary advantages. Some viruses also encode special DNA polymerases, such as Hepatitis B virus DNA polymerase. These may selectively replicate viral DNA through a variety of mechanisms. Retroviruses encode an unusual DNA polymerase called reverse transcriptase, which is an RNA-dependent DNA polymerase (RdDp). It polymerizes DNA from a template of RNA. DNA polymerase families Based on sequence homology, DNA polymerases can be further subdivided into seven different families: A, B, C, D, X, Y, and RT. Family A Polymerases contain both replicative and repair polymerases. Replicative members from this family include the extensively-studied T7 DNA polymerase, as well as the eukaryotic mitochondrial DNA Polymerase γ. Among the repair polymerases are Escherichia coli DNA pol I, Thermus aquaticus pol I, and Bacillus stearothermophilus pol I. These repair polymerases are involved in excision repair and processing of Okazaki fragments generated during lagging strand synthesis. Family B Polymerases mostly contain replicative polymerases and include the major eukaryotic DNA polymerases α, δ, ε, (see Greek letters) and also DNA polymerase ζ. Family B also includes DNA polymerases encoded by some bacteria and bacteriophages, of which the best-characterized are from T4, Phi29, and RB69 bacteriophages. These enzymes are involved in both leading and lagging strand synthesis during replication. A hallmark of the B family of polymerases is their highly faithful DNA synthesis during replication. While many have an intrinsic 3'-5' proofreading exonuclease activity, eukaryotic DNA polymerases α and ζ are two examples of B family polymerases lacking this proofreading activity. Family C Polymerases are the primary bacterial chromosomal replicative enzymes. DNA Polymerase III alpha subunit from E. coli is the catalytic subunit [1] and possesses no known nuclease activity. A separate subunit, the epsilon subunit, possesses the 3'-5' exonuclease activity used for editing during chromosomal replication. Recent research has classified Family C polymerases as a subcategory of Family X. Family D Polymerases are still not very well characterized. All known examples are found in the Euryarchaeota subdomain of Archaea and are thought to be replicative polymerases. Family X Contains the well-known eukaryotic polymerase pol β, as well as other eukaryotic polymerases such as pol σ, pol λ, pol μ, and terminal deoxynucleotidyl transferase (TdT). Pol β is required for short-patch base excision repair, a DNA repair pathway that is essential for repairing abasic sites. Pol λ and Pol μ are involved in non-homologous end-joining, a mechanism for rejoining DNA double-strand breaks. TdT is expressed only in lymphoid tissue, and adds "n nucleotides" to double-strand breaks formed during V(D)J recombination to promote immunological diversity. The yeast Saccharomyces cerevisiae has only one Pol X polymerase, Pol4, which is involved in non-homologous end-joining. Family Y Polymerases differ from others in having a low fidelity on undamaged templates and in their ability to replicate through damaged DNA. Members of this family are hence called translesion synthesis (TLS) polymerases. Depending on the lesion, TLS polymerases can bypass the damage in an error-free or error-prone fashion, the latter resulting in elevated mutagenesis. Xeroderma pigmentosum variant (XPV) patients for instance have mutations in the gene encoding Pol η (eta), which is error-free for UV-lesions. In XPV patients, alternative error-prone polymerases, e.g., Polζ (zeta) (polymerase ζ is a B Family polymerase a complex of the catalytic subunit REV3L with Rev7, which associates with Rev1[2]), are thought to be involved in mistakes that result in the cancer predisposition of these patients. Other members in humans are Pol ι (iota), Pol κ (kappa), and Rev1 (terminal deoxycytidyl transferase). In E. coli, two TLS polymerases, Pol IV (DINB) and PolV (UmuD'2C), are known. Family RT The reverse transcriptase family contains examples from both retroviruses and eukaryotic polymerases. The eukaryotic polymerases are usually restricted to telomerases. These polymerases use an RNA template to synthesize the DNA strand. Prokaryotic DNA polymerases Bacteria have 5 known DNA polymerases: * Pol I: implicated in DNA repair; has 5'->3' (Polymerase) activity and both 3'->5' exonuclease (Proofreading) and 5'->3' exonuclease activity (RNA Primer removal).
Eukaryotes have at least 15 DNA Polymerases:[3] * Pol α (also called RNA primase): forms a complex with a small catalytic (PriS) and a large noncatalytic (PriL) subunit[4], with the Pri subunits acting as a primase (synthesizing an RNA primer), and then with DNA Pol α elongating that primer with DNA nucleotides. After around 20 nucleotides[5] elongation is taken over by Pol ε (on the leading strand) and δ (on the lagging strand). None of the eukaryotic polymerases can remove primers (5'->3' exonuclease activity); that function is carried out by other enzymes. Only the polymerases that deal with the elongation (γ, δ and ε) have proofreading ability (3'->5' exonuclease). See also * Polymerase chain reaction
1. ^ Lamers, M.; Georgescu, R.; Lee, S.; O'Donnell, M.; Kuriyan, J. (2006). "Crystal structure of the catalytic alpha subunit of E. coli replicative DNA polymerase III". Cell 126 (5): 881–892. doi:10.1016/j.cell.2006.07.028. PMID 16959568. edit
* Burgers P, Koonin E, Bruford E et al. (2001). "Eukaryotic DNA polymerases: proposal for a revised nomenclature". J. Biol. Chem. 276 (47): 43487–90. doi:10.1074/jbc.R100056200. PMID 11579108. Retrieved from "http://en.wikipedia.org/"
|