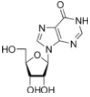
Inosine is a nucleoside that is formed when hypoxanthine is attached to a ribose ring (also known as a ribofuranose) via a β-N9-glycosidic bond. Inosine is commonly found in tRNAs and is essential for proper translation of the genetic code in wobble base pairs. Knowledge of inosine metabolism has led to advances in immunotherapy in recent decades. Inosine monophosphate is oxidised by the enzyme inosine monophosphate dehydrogenase yielding xanthosine monophosphate, a key precursor in purine metabolism. Mycophenolate mofetil is an anti-metabolite, anti-proliferative drug that acts as an inhibitor of inosine monophosphate dehydrogenase. It is used in the treatment of a variety of autoimmune diseases including Wegener's granulomatosis because the uptake of purine by actively dividing B cells can exceed 8 times that of normal body cells and therefore this set of white cells (which cannot operate purine salvage pathways) is selectively targeted by the purine deficiency resulting from IMD inhibition.
Adenine is converted to adenosine or inosine monophosphate (IMP), either of which in turn is converted into inosine (I), which pairs with Adenine (A), cytosine (C) and uracil (U). Purine nucleoside phosphorylase intraconverts inosine and hypoxanthine. Inosine is also an intermediate in a chain of purine nucleotides reactions required for muscle movements. It was tried in the seventies in eastern countries for improving athletic performance. Nevertheless the clinical trials for this purpose showed no improvement.[1] It has been shown that inosine has neuroprotective properties. It has been proposed for spinal cord injury;[2] because it improves axonal rewiring, and for administration after stroke, because observation has shown that axonal re-wiring is encouraged.[3] It is currently in phase II trials for multiple sclerosis (MS).[4] It produces uric acid after ingestion, which is a natural antioxidant and a peroxynitrite scavenger, which can suggest possible benefit in multiple sclerosis[5] (peroxynitrite has been correlated with the axons degeneration[1]). Alseres Pharmaceuticals (named Boston Life Sciences when patent was granted) patented the treatment for stroke[2] and is currently investigating the drug in the MS setting.[6] In the Anatomical Therapeutic Chemical Classification System, it is classified as an antiviral.[7] Biotechnology When designing primers for polymerase chain reaction, inosine is useful in that it will indiscriminately pair with adenine, thymine, or cytosine. This allows for design of primers that span a single nucleotide polymorphism, without the polymorphism disrupting the primer's annealing efficiency. Fitness Despite a lack of clinical evidence that it improves muscle development, inosine remains an ingredient in some fitness supplements. Feeding Stimulant Inosine has also been found to be an important feed stimulant by itself or in combination with certain amino acids in some species of farmed fish. For example, inosine and inosine-5-monophosphate have been reported as specific feeding stimulants for turbot fry, (Scophthalmus maximus) [8] and yellowtail, (Seriola quinqueradiata).[9] The main problem of using inosine and/or inosine-5-monophosphate as feeding attractants is their high cost. However, their use may be economically justified within larval feeds for marine fish larvae during the early weaning period since the total quantity of feed consumed is relatively low. Experimental NOV-205 manufactured by Novelos Therapeutics is A new class of Antiviral medication that is approved for use in Russia under the tradename of "Molixan" and currently being tested in the USA under FDA clinical studies. See also * Inosine monophosphate dehydrogenase
1. ^ McNaughton L, Dalton B, Tarr J (1999). "Inosine supplementation has no effect on aerobic or anaerobic cycling performance". International journal of sport nutrition 9 (4): 333–44. PMID 10660865.
* PDR health study Retrieved from "http://en.wikipedia.org/"
|