Small interfering RNA (siRNA), sometimes known as short interfering RNA or silencing RNA, is a class of double-stranded RNA molecules, 20-25 nucleotides in length, that play a variety of roles in biology. Most notably, siRNA is involved in the RNA interference (RNAi) pathway, where it interferes with the expression of a specific gene. In addition to their role in the RNAi pathway, siRNAs also act in RNAi-related pathways, e.g., as an antiviral mechanism or in shaping the chromatin structure of a genome; the complexity of these pathways is only now being elucidated. siRNAs were first discovered by David Baulcombe's group at the Sainsbury Laboratory in Norwich, England, as part of post-transcriptional gene silencing (PTGS) in plants. The group published their findings in Science in a paper titled "A species of small antisense RNA in posttranscriptional gene silencing in plants".[1] Shortly thereafter, in 2001, synthetic siRNAs were shown to be able to induce RNAi in mammalian cells by Thomas Tuschl, and colleagues in a paper published in Nature.[2] This discovery led to a surge in interest in harnessing RNAi for biomedical research and drug development.
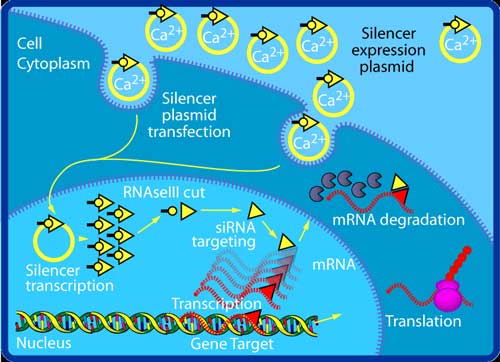
Mediating RNA interference in cultured mammalian cells. Structure siRNAs have a well-defined structure: a short (usually 21-nt) double strand of RNA (dsRNA) with 2-nt 3' overhangs on either end: Each strand has a 5' phosphate group and a 3' hydroxyl (-OH) group. This structure is the result of processing by dicer, an enzyme that converts either long dsRNAs or small hairpin RNAs into siRNAs.[3] siRNAs can also be exogenously (artificially) introduced into cells by various transfection methods to bring about the specific knockdown of a gene of interest. Essentially any gene of which the sequence is known can thus be targeted based on sequence complementarity with an appropriately tailored siRNA. This has made siRNAs an important tool for gene function and drug target validation studies in the post-genomic era. RNAi induction using siRNAs or their biosynthetic precursors
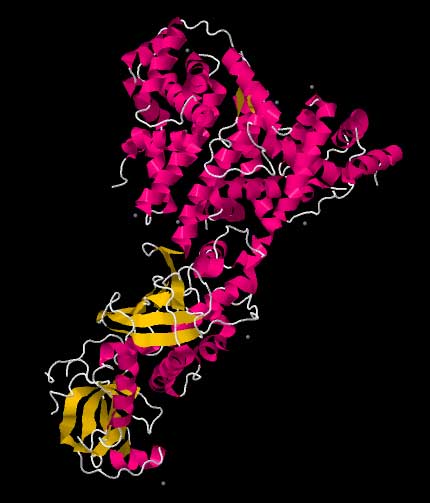
One molecule of the Dicer-homolog protein from Giardia intestinalis . Dicer is an RNase that cleaves long double-stranded RNA molecules into short interfering RNAs (siRNAs) as a first step in the RNA interference response, and also initiates the formation of the RNA-induced silencing complex (RISC). Transfection of an exogenous siRNA can be problematic because the gene knockdown effect is only transient, particularly in rapidly dividing cells. One way of overcoming this challenge is to modify the siRNA in such a way as to allow it to be expressed by an appropriate vector, e.g., a plasmid. This is done by the introduction of a loop between the two strands, thus producing a single transcript, which can be processed into a functional siRNA. Such transcription cassettes typically use an RNA polymerase III promoter (e.g., U6 or H1), which usually directs the transcription of small nuclear RNAs (snRNAs) (U6 is involved in gene splicing; H1 is the RNase component of human RNase P). It is assumed (although not known for certain) that the resulting siRNA transcript is then processed by Dicer. RNA activation It has recently been found that dsRNA can also activate gene expression, a mechanism that has been termed "small RNA-induced gene activation" or RNAa. It has been shown that dsRNAs targeting gene promoters induce potent transcriptional activation of associated genes. RNAa was demonstrated in human cells using synthetic dsRNAs, termed "small activating RNAs" (saRNAs). It is currently not known whether RNAa is conserved in other organisms.[4] Challenges: avoiding nonspecific effects Because RNAi intersects with a number of other pathways, it is not surprising that on occasion nonspecific effects are triggered by the experimental introduction of an siRNA. When a mammalian cell encounters a double-stranded RNA such as an siRNA, it may mistake it as a viral by-product and mount an immune response. Furthermore, because structurally related microRNAs modulate gene expression largely via incomplete complementarity base pair interactions with a target mRNA, the introduction of an siRNA may cause unintended off-targeting. Innate immunity Introduction of too much siRNA can result in nonspecific events due to activation of innate immune responses. Most evidence to date suggests that this is probably due to activation of the dsRNA sensor PKR, although retinoic acid inducible gene I (RIG-I) may also be involved. The induction of cytokines via toll-like receptor 7 (TLR7) has also been described. One promising method of reducing the nonspecific effects is to convert the siRNA into a microRNA. MicroRNAs occur naturally, and by harnessing this endogenous pathway it should be possible to achieve similar gene knockdown at comparatively low concentrations of resulting siRNAs. This should minimize nonspecific effects. Off-targeting Off-targeting is another challenge to the use of siRNAs as a gene knockdown tool. Here, genes with incomplete complementarity are inadvertently downregulated by the siRNA (effectively, the siRNA acts as a miRNA), leading to problems in data interpretation and potential toxicity. This, however, can be partly addressed by designing appropriate control experiments, and siRNA design algorithms are currently being developed to produce siRNAs free from off-targeting. Genome-wide expression analysis, e.g., by microarray technology, can then be used to verify this and further refine the algorithms. A 2006 paper from the laboratory of Dr. Khvorova implicates 6- or 7-basepair-long stretches from position 2 onward in the siRNA matching with 3'UTR regions in off-targeted genes.[5] Possible therapeutic applications and challenges Given the ability to knock down essentially any gene of interest, RNAi via siRNAs has generated a great deal of interest in both basic[6] and applied biology. There are an increasing number of large-scale RNAi screens that are designed to identify the important genes in various biological pathways. Because disease processes also depend on the activity of multiple genes, it is expected that in some situations turning off the activity of a gene with an siRNA could produce a therapeutic benefit. However, applying RNAi via siRNAs to living animals, especially humans, poses many challenges. Experimentally, siRNAs show different effectiveness in different cell types in a manner as yet poorly understood: some cells respond well to siRNAs and show a robust knockdown, whereas others show no such knockdown (even despite efficient transfection). Phase I results of the first two therapeutic RNAi trials (indicated for age-related macular degeneration, aka AMD) reported at the end of 2005 that siRNAs are well tolerated and have suitable pharmacokinetic properties.[7] See also * Esirna
1. ^ Hamilton A, Baulcombe D (1999). "A species of small antisense RNA in posttranscriptional gene silencing in plants". Science 286 (5441): 950–2. doi:10.1126/science.286.5441.950. PMID 10542148.
* Hannon G, Rossi J (2004). "Unlocking the potential of the human genome with RNA interference". Nature 431 (7006): 371–8. doi:10.1038/nature02870. PMID 15372045. http://www.nature.com/nature/journal/v431/n7006/full/nature02870.html.
* HuSiDa: Human siRNA Database
Retrieved from "http://en.wikipedia.org/"
|