A dye laser is a laser which uses an organic dye as the lasing medium, usually as a liquid solution. Compared to gases and most solid state lasing media, a dye can usually be used for a much wider range of wavelengths. The wide bandwidth makes them particularly suitable for tunable lasers and pulsed lasers. Moreover, the dye can be replaced by another type in order to generate different wavelengths with the same laser, although this usually requires replacing other optical components in the laser as well.
Dye lasers were independently discovered by P. P. Sorokin and F. P. Schäfer (and colleagues) in 1966.[1][2]
In addition to the usual liquid state, dye lasers are also available as solid state dye lasers (SSDL). SSDL use dye-doped organic matrices as gain medium.
Construction
A dye laser consists of an organic dye mixed with a solvent, which may be circulated through a dye cell, or streamed through open air using a dye jet. A high energy source of light is needed to "pump" the liquid beyond its lasing threshold. A fast discharge flashlamp or an external laser is usually used for this purpose. Mirrors are also needed to oscillate the light produced by the dye’s fluorescence, which is amplified with each pass through the liquid. The output mirror is normally around 80% reflective, while all other mirrors are usually more than 99% reflective. The dye solution is usually circulated at high speeds, to help avoid triplet absorption and to decrease degradation of the dye. A prism or diffraction grating is usually mounted in the beam path, to allow tuning of the beam.
A dielectric mirror used in a dye laser.
Because the liquid medium of a dye laser can fit any shape, there are a multitude of different configurations that can be used. A Fabry–Pérot laser cavity is usually used for flashlamp pumped lasers, which consists of two mirrors, which may be flat or curved, mounted parallel to each other with the laser medium in between. The dye cell is usually side-pumped, with one or more flashlamps running parallel to the dye cell in a reflector cavity. The reflector cavity is often water cooled, to prevent thermal shock in the dye caused by the large amounts of near-infrared radiation which the flashlamp produces. Axial pumped lasers have a hollow, annular-shaped flashlamp that surrounds the dye cell, which has lower inductance for a shorter flash, and improved transfer efficiency. Coaxial pumped lasers have an annular dye cell that surrounds the flash lamp, for even better transfer efficiency, but have a lower gain due to diffraction losses. Flash pumped lasers can only be used for pulsed output.[3][4][5]
Multiple prisms are often used to tune the output of a dye laser
A ring laser design is often chosen for continuous operation, although a Fabry–Pérot design is sometimes used. In a ring laser, the mirrors of the laser are positioned to allow the beam to travel in a circular path. The dye cell, or cuvette, is usually very small. Sometimes a dye jet is used to help avoid reflection losses. The dye is usually pumped with an external laser, such as a nitrogen, excimer, or frequency doubled Nd:YAG laser. The liquid is circulated at very high speeds, to prevent triplet absorption from cutting off the beam.[6] Unlike Fabry–Pérot cavities, a ring laser does not generate standing waves which cause spatial hole burning, a phenomenon where energy becomes trapped in unused portions of the medium between the crests of the wave. This leads to a better gain from the lasing medium.[7][8]
Operation
The dyes used in these lasers contain rather large organic molecules which fluoresce when exposed to the proper frequency of light. Dyes will emit stimulated radiation when the molecules are in their singlet state. In this state, the molecules emit light via fluorescence, and the dye is quite clear to the lasing wavelength. Within a microsecond, or less, the molecules will change to their triplet state. In the triplet state, light is emitted via phosphorescence, and the molecules begin to absorb the lasing wavelength, making the dye opaque. Liquid dyes also have an extremely high lasing threshold. Flashlamp pumped lasers need a flash with an extremely short duration, to deliver the large amounts of energy necessary to bring the dye past threshold before triplet absorption overcomes singlet emission. Dye lasers with an external pump laser can direct enough energy of the proper wavelength into the dye with a relatively small amount of input energy, but the dye must be circulated at high speeds to keep the triplet molecules out of the beam path.[9]
A cuvette used in a dye laser
Since organic dyes tend to degrade under the influence of light, the dye solution is normally circulated from a large reservoir[10]. The dye solution can be flowing through a cuvette, i.e., a glass container, or be as a dye jet, i.e., as a sheet-like stream in open air from a specially-shaped nozzle. With a dye jet, one avoids reflection losses from the glass surfaces and contamination of the walls of the cuvette. These advantages come at the cost of a more-complicated alignment. Dye lasers emission is inherently broad. In order to produce narrow bandwidth tuning these lasers use many types of cavities and resonators which include gratings, prisms, and etalons[11].
Liquid dyes are very high gain laser mediums. The beam only needs to make a few passes through the liquid for high gains in power, and hence, the high transmittance of the output coupler. This high gain nature also leads to very high losses, as any reflections generated by the dye cell walls, or flashlamp reflector, will dramatically reduce the amount of energy available to the beam. Pumping cavities are often coated, anodized, or otherwise made of a material that will absorb the lasing wavelength while effectively reflecting the pumping energy.[9]
Chemicals used

Rhodamine 6G Chloride powder; mixed with methanol; emitting yellow light under the influence of a green laser
Some of the dyes are rhodamine, fluorescein, coumarin, stilbene, umbelliferone, tetracene, malachite green, and others. While some dyes are actually used in food coloring, most dyes are very toxic, and often carcinogenic. Many dyes, such as rhodamine 6G, (in its chloride form), can be very corrosive to all metals except stainless steel.
A wide variety of solvents can be used, although some dyes will dissolve better in some solvents than in others. Some of the solvents used are water, glycol, ethanol, methanol, hexane, cyclohexane, cyclodextrin, and many others. Solvents are often highly toxic, and can sometimes be absorbed directly through the skin, or through inhaled vapors. Many solvents are also extremely flammable.
Adamantane is added to some dyes to prolong their life.
Cycloheptatriene and cyclooctatetraene (COT) can be added as triplet quenchers for rhodamine G, increasing the laser output power. Output power of 1.4 kilowatt at 585 nm was achieved using Rhodamine 6G with COT in methanol-water solution.
Excitation lasers
As already mentioned flashlamps, and several types of lasers, can be used to optically pump dye lasers. A partial list of excitation lasers include[12]:
* Copper vapor lasers
* Diode lasers
* Excimer lasers
* Nd:YAG lasers (mainly second and third harmonics)
* Nitrogen lasers
* Ruby lasers
* Argon ion lasers in the CW regime
* Krypton ion lasers in the CW regime
Ultra-short optical pulses
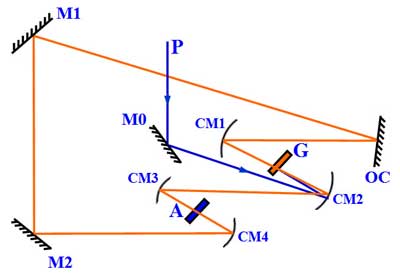
A ring dye laser. P-pump laser beam; G-gain dye jet; A-saturable absorber dye jet; M0, M1, M2-planar mirrors; OC–output coupler; CM1 to CM4-curved mirrors.
R. L. Fork, B. I. Greene, and C. V. Shank have demonstrated, in 1981, the generation of ultra-short laser pulse using a ring-dye laser (or dye laser exploiting colliding pulse mode-locking). Such kind of laser is capable of generating laser pulses of ~ 0.1 ps duration.[13]
Applications
Dye lasers are very versatile. In addition to their recognized wavelength agility these lasers can offer very large pulsed energies or very high average powers. Flashlamp-pumped dye lasers have been shown to yield hundreds of Joules per pulse and copper-laser-pumped dye lasers are known to yield average powers in the kilowatt regime.[14]
Dye lasers are used in many applications including:
* astronomy (as laser guide stars),
* atomic vapor laser isotope separation[15]
* manufacturing[16]
* medicine
* spectroscopy.[17]
In laser medicine these lasers are applied in several areas,[18][19] including dermatology where they are used to make skin tone more even. The wide range of wavelengths possible allows very close matching to the absorption lines of certain tissues, such as melanin or hemoglobin, while the narrow bandwidth obtainable helps reduce the possibility of damage to the surrounding tissue. They are used to treat port-wine stains and other blood vessel disorders, scars and kidney stones. They can be matched to a variety of inks for tattoo removal, as well as a number of other applications.[20]
In spectroscopy, dye lasers can be used to study the absorption and emission spectra of various materials. Their tunability, (from the near-infrared to the near-ultraviolet), narrow bandwidth, and high intensity allows a much greater diversity than other light sources. The variety of pulse widths, from ultra-short, femto-second pulses to continuous-wave operation, makes them suitable for a wide range of applications, from the study of fluorescent lifetimes and semiconductor properties to lunar laser ranging experiments.[21]
References
1. ^ F. P. Schäfer (Ed.), Dye Lasers (Springer-Verlag, Berlin, 1990).
2. ^ F. J. Duarte and L. W. Hillman (Eds.), Dye Laser Principles (Academic, New York, 1990).
3. ^ Design and Analysis of Flashlamp Systems for Pumping Organic Dye Lasers – J. F. Holzrichter and A. L. Schawlow. Annals of the New York Academy of Sciences
4. ^ Simmer-Enhanced Flashlamp Pumped Dye Laser – T.K. Yee, B. Fan and T.K. Gustafson. Applied Optics – Vol. 18, No. 8
5. ^ http://members.misty.com/don/xeguide.html#eg
6. ^ http://www.repairfaq.org/sam/lasercdy.htm
7. ^ http://www.rp-photonics.com/spatial_hole_burning.html
8. ^ Laser fundamentals by William Thomas Silfvast – Cambridge University Press 1996 Page 397-399
9. ^ a b "Principles of Lasers", by Orazio Svelto
10. ^ F. P. Schäfer and K. H. Drexhage, Dye Lasers., 2nd rev. ed., vol. 1, Berlin ; New York: Springer-Verlag, 1977
11. ^ F. J. Duarte and L. W. Hillman, Dye Laser Principles (Academic, New York, 1990) Chapter 4.
12. ^ F. J. Duarte and L. W. Hillman (Eds.), Dye Laser Principles (Academic, New York, 1990) Chapters 5 and 6.
13. ^ R. L. Fork, B. I. Greene, and C. V. Shank (1981), “Generation of optical pulses shorter than 0.1 psec by colliding pulse mode locking,” Applied Physics Letters, 38: 671–672.
14. ^ F. J. Duarte (Ed.), High Power Dye Lasers (Springer-Verlag, Berlin,1991).
15. ^ M. A. Akerman, Dye laser isotope separation, in Dye Laser Principles, F. J. Duarte and L. W. Hillman (Eds.)(Academic, New York, 1990) Chapter 9.
16. ^ D. Klick, Industrial applications of dye lasers, in Dye Laser Principles, F. J. Duarte and L. W. Hillman (Eds.)(Academic, New York, 1990) Chapter 8.
17. ^ W. Demtröder, Laser Spectroscopy, 3rd Ed. (Springer, 2003).
18. ^ L. Goldman, Dye lasers in medicine, in Dye Laser Principles , F. J. Duarte and L. W. Hillman, Eds. (Academic, New York, 1990) Chapter 10.
19. ^ A. Costela et al., Medical applications of dye lasers, in Tunable Laser Applications, F. J. Duarte (Ed.), 2nd Ed. (CRC, New York, 2009) Chapter 8
20. ^ Tunable Laser Applications By Frank J. Duarte – CRC Press 2009 Page 227-235
21. ^ The Laser Guidebook By Jeff Hecht – McGraw Hill 1992 Page 294
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License

