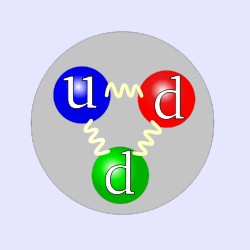
The simplified quark structure of the neutron.
In physics, the neutron is a subatomic particle with no net electric charge and a mass of 939.573 MeV/c² or 1.008 664 915 (78) u (1.6749 × 10−27 kg, slightly more than a proton). Its spin is ½. Its antiparticle is called the antineutron. The neutron, along with the proton, is a nucleon.
The nuclei of all atoms consist of protons and neutrons, except the lightest isotope of hydrogen which has only a single proton. The number of protons defines the type of element the atom forms. The number of neutrons determines the isotope of an element, therefore isotopes are atoms of the same element (i.e. atomic number) but differing atomic masses due to a different number of neutrons. For example, the carbon-12 isotope has 6 protons and 6 neutrons, while the carbon-14 isotope has 6 protons and 8 neutrons.
A neutron consists of two down quarks and one up quark. Since it has three quarks, it is classified as a baryon.
Composition: one up, two down
Family: Fermion
Group: Quark
Interaction: Gravity, Electromagnetic, Weak, Strong
Antiparticle: Antineutron
Discovered: James Chadwick[1] (1932)
Symbol: n
Mass: 1.674 927 29(28) × 10−27kg
939.565 560(81) MeV/c²
1.008665 u
Electric charge: 0 C
Spin: ½
Neutron stability and beta decay
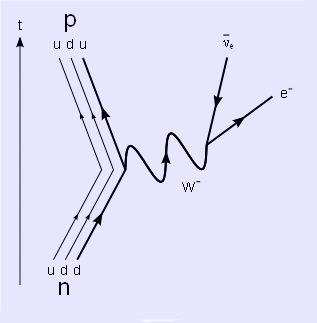
The Feynman diagram of the neutron beta decay process
Outside the nucleus, free neutrons are unstable and have a mean lifetime of 885.7±0.8 seconds (about 15 minutes), decaying by emission of a negative electron and antineutrino to become a proton:[2] ![]() . This decay mode, known as beta decay, can also transform the character of neutrons within unstable nuclei.
. This decay mode, known as beta decay, can also transform the character of neutrons within unstable nuclei.
Inside of a bound nucleus, protons can also transform via beta decay into neutrons. In this case, the transformation may occur by emission of a positron (antielectron) and neutrino (instead of an antineutrino): ![]() The transformation of a proton to a neutron inside of a nucleus is also possible through electron capture:
The transformation of a proton to a neutron inside of a nucleus is also possible through electron capture: ![]() . Positron capture by neutrons in nuclei that contain an excess of neutrons is also possible, but is hindered due to the fact positrons are repelled by the nucleus, and furthermore, quickly annihilate when they encounter negative electrons.
. Positron capture by neutrons in nuclei that contain an excess of neutrons is also possible, but is hindered due to the fact positrons are repelled by the nucleus, and furthermore, quickly annihilate when they encounter negative electrons.
When bound inside of a nucleus, the instability of a single neutron to beta decay is balanced against the instability that would be acquired by the nucleus as a whole if an additional proton were to participate in repulsive interactions with the other protons that are already present in the nucleus. As such, although free neutrons are unstable, bound neutrons are not necessarily so. The same reasoning explains why protons, which are stable in empty space, may transform into neutrons when bound inside of a nucleus.
Beta decay and electron capture are types of radioactive decay and are both governed by the weak interaction.
Interactions
The neutron interacts through all four fundamental interactions: the electromagnetic, weak nuclear, strong nuclear and gravitational interactions.
Although the neutron has zero net charge, it may interact electromagnetically in two ways: first, the neutron has a magnetic moment of the same order as the proton (see neutron magnetic moment);[3] second, it is composed of electrically charged quarks. Thus, the electromagnetic interaction is primarily important to the neutron in deep inelastic scattering and in magnetic interactions.
The neutron experiences the weak interaction through beta decay into a proton, electron and electron antineutrino. It experiences the gravitational force as does any energetic body; however, gravity is so weak that it may be neglected in particle physics experiments.
The most important force to neutrons is the strong interaction. This interaction is responsible for the binding of the neutron's three quarks into a single particle. The residual strong force is responsible for the binding of neutrons and protons together into nuclei. This nuclear force plays the leading role when neutrons pass through matter. Unlike charged particles or photons, the neutron cannot lose energy by ionizing atoms. Rather, the neutron goes on its way unchecked until it makes a head-on collision with an atomic nucleus. For this reason, neutron radiation is extremely penetrating.
Detection
Main article: neutron detection
The common means of detecting a charged particle by looking for a track of ionization (such as in a cloud chamber) does not work for neutrons directly. Neutrons that elastically scatter off atoms can create an ionization track that is detectable, but the experiments are not as simple to carry out; other means for detecting neutrons, consisting of allowing them to interact with atomic nuclei, are more commonly used.
A common method for detecting neutrons involves converting the energy released from such reactions into electrical signals. The nuclides 3He, 6Li, 10B, 233U, 235U, 237Np and 239Pu are useful for this purpose. A good discussion on neutron detection is found in chapter 14 of the book Radiation Detection and Measurement by Glenn F. Knoll (John Wiley & Sons, 1979).
Uses
The neutron plays an important role in many nuclear reactions. For example, neutron capture often results in neutron activation, inducing radioactivity. In particular, knowledge of neutrons and their behavior has been important in the development of nuclear reactors and nuclear weapons.
Cold, thermal and hot neutron radiation is commonly employed in neutron scattering facilities, where the radiation is used in a similar way one uses X-rays for the analysis of condensed matter. Neutrons are complementary to the latter in terms of atomic contrasts by different scattering cross sections; sensitivity to magnetism; energy range for inelastic neutron spectroscopy; and deep penetration into matter.
The development of "neutron lenses" based on total internal reflection within hollow glass capillary tubes or by reflection from dimpled aluminum plates has driven ongoing research into neutron microscopy and neutron/gamma ray tomography.[4][5][6]
One use of neutron emitters is the detection of light nuclei, particularly the hydrogen found in water molecules. When a fast neutron collides with a light nucleus, it loses a large fraction of its energy. By measuring the rate at which slow neutrons return to the probe after reflecting off of hydrogen nuclei, a neutron probe may determine the water content in soil.
Sources
Due to the fact that free neutrons are unstable, they can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or accelerator collisions). Free neutron beams are obtained from neutron sources by neutron transport. For access to intense neutron sources, researchers must go to specialist facilities, such as the ISIS facility in the UK, which is currently the world's most intense pulsed neutron and muon source.
Neutrons' lack of total electric charge prevents engineers or experimentalists from being able to steer or accelerate them. Charged particles can be accelerated, decelerated, or deflected by electric or magnetic fields. However, these methods have no effect on neutrons except for a small effect of a magnetic field because of the neutron's magnetic moment.
In 1930 Walther Bothe and H. Becker in Germany found that if the very energetic alpha particles emitted from polonium fell on certain light elements, specifically beryllium, boron, or lithium, an unusually penetrating radiation was produced. At first this radiation was thought to be gamma radiation, although it was more penetrating than any gamma rays known, and the details of experimental results were very difficult to interpret on this basis. The next important contribution was reported in 1932 by Irène Joliot-Curie and Frédéric Joliot in Paris. They showed that if this unknown radiation fell on paraffin or any other hydrogen-containing compound it ejected protons of very high energy. This was not in itself inconsistent with the assumed gamma ray nature of the new radiation, but detailed quantitative analysis of the data became increasingly difficult to reconcile with such a hypothesis. Finally, in 1932 the physicist James Chadwick in England performed a series of experiments showing that the gamma ray hypothesis was untenable. He suggested that in fact the new radiation consisted of uncharged particles of approximately the mass of the proton, and he performed a series of experiments verifying his suggestion.[7] Such uncharged particles were eventually called neutrons, apparently from the Latin root for neutral and the Greek ending -on (by imitation of electron and proton).
Anti-neutron
Main article: antineutron
The antineutron is the antiparticle of the neutron. It was discovered by Bruce Cork in the year 1956, a year after the antiproton was discovered.
CPT-symmetry puts strong constraints on the relative properties of particles and antiparticles and, therefore, is open to stringent tests. The fractional difference in the masses of the neutron and antineutron is (9±5)×10−5. Since the difference is only about 2 standard deviations away from zero, this does not give any convincing evidence of CPT-violation.[3]
Current developments
Electric dipole moment
An experiment at the Institut Laue-Langevin has attempted to measure an electric dipole, or separation of charges, within the neutron, and is consistent with an electric dipole moment of zero. These results are important in developing theories that go beyond the Standard Model, but are inconsistent with it due to the lack of explanation of the fundamental interactions.[8][9]
Tetraneutrons
Main article: tetraneutron
The existence of stable clusters of four neutrons, or tetraneutrons, has been hypothesised by a team led by Francisco-Miguel Marqués at the CNRS Laboratory for Nuclear Physics based on observations of the disintegration of beryllium-14 nuclei. This is particularly interesting, because current theory suggests that these clusters should not be stable.
Protection
Exposure to neutrons can be hazardous, since the interaction of neutrons with molecules in the body can cause disruption to molecules and atoms, and can also cause reactions which give rise to other forms of radiation (such as protons). The normal precautions of radiation protection apply: avoid exposure, stay as far from the source as possible, and keep exposure time to a minimum. Some particular thought must be given to how to protect from neutron exposure, however. For other types of radiation, e.g. alpha particles, beta particles, or gamma rays, material of a high atomic number and with high density make for good shielding; frequently lead is used. However, this approach will not work with neutrons, since the absorption of neutrons does not increase straightforwardly with atomic number, as it does with alpha, beta, and gamma radiation. Instead one needs to look at the particular interactions neutrons have with matter (see the section on detection above). For example, hydrogen rich materials are often used to shield against neutrons, since ordinary hydrogen both scatters and slows neutrons. This often means that simple concrete blocks or even paraffin-loaded plastic blocks afford better protection from neutrons than do far more dense materials. After slowing, neutrons may then be absorbed with an isotope which has high affinity for slow neutrons without causing secondary capture-radiation, such as lithium-6.
Hydrogen-rich ordinary water effects neutron absorption in nuclear fission reactors: usually neutrons are so strongly absorbed by normal water that fuel-enrichement with fissionable isotope, is required. The deuterium in heavy water has a very much lower absorption affinity for neutrons than does protium (normal light hydrogen). Deuterium is therefore used in CANDU-type reactors, in order to slow ("moderate") neutron velocity, so that they are more effective at causing nuclear fission, without capturing them.
Atomic Structure: Discovery of the Neutron
See also
Fields concerning neutrons
* chemistry
* Neutron Detection
* Neutron Scattering
Types of neutrons
* fast neutron
* free neutron
* thermal neutron
* neutron radiation and the Sievert radiation scale
* neutron temperature, used to classify neutron types
Objects containing neutrons
* the nuclei of atoms with the exception of protium, the most common isotope of hydrogen (and as a result, all ordinary matter with the exception of hydrogen)
* dineutron
* tetraneutron
* neutronium
* neutron star
Neutron sources
* Neutron sources
* Neutron generator
Processes involving neutrons
* neutron transport
* neutron diffraction
* neutron bomb
References
1. ^ 1935 Nobel Prize in Physics
2. ^ Particle Data Group Summary Data Table on Baryons
3. ^ a b Particle Data Group's Review of Particle Physics 2006
4. ^ Nature 357, 390-391 (04 June 1992); doi:10.1038/357390a0
5. ^ Physorg.com, "New Way of 'Seeing': A 'Neutron Microscope'"
6. ^ NASA.gov: "NASA Develops a Nugget to Search for Life in Space"
7. ^ Chadwick, J., "Possible Existence of a Neutron," Nature, 1932
8. ^ FRONTIERS, "Particle physics chills out", retrieved 25 November 2007
9. ^ Neutron Electric Dipole Moment experiment's web page, retrieved 25 November 2007
Retrieved from "http://en.wikipedia.org/"
All text is available under the terms of the GNU Free Documentation License

