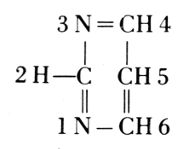SMITHSONIAN INSTITUTION
UNITED STATES NATIONAL MUSEUM
BULLETIN 240
SMITHSONIAN PRESS
MUSEUM OF HISTORY AND TECHNOLOGY
Contributions
From the
Museum
of History and
Technology
Papers 34-44
On Science and Technology
SMITHSONIAN INSTITUTION · WASHINGTON, D.C. 1966
Publications of the United States National Museum
The scholarly and scientific publications of the United States National Museum include two series, Proceedings of the United States National Museum and United States National Museum Bulletin.
In these series, the Museum publishes original articles and monographs dealing with the collections and work of its constituent museums—The Museum of Natural History and the Museum of History and Technology—setting forth newly acquired facts in the fields of anthropology, biology, history, geology, and technology. Copies of each publication are distributed to libraries, to cultural and scientific organizations, and to specialists and others interested in the different subjects.
The Proceedings, begun in 1878, are intended for the publication, in separate form, of shorter papers from the Museum of Natural History. These are gathered in volumes, octavo in size, with the publication date of each paper recorded in the table of contents of the volume.
In the Bulletin series, the first of which was issued in 1875, appear longer, separate publications consisting of monographs (occasionally in several parts) and volumes in which are collected works on related subjects. Bulletins are either octavo or quarto in size, depending on the needs of the presentation. Since 1902 papers relating to the botanical collections of the Museum of Natural History have been published in the Bulletin series under the heading Contributions from the United States National Herbarium, and since 1959, in Bulletins titled “Contributions from the Museum of History and Technology,” have been gathered shorter papers relating to the collections and research of that Museum.
The present collection of Contributions, Papers 34-44, comprises Bulletin 240. Each of these papers has been previously published in separate form. The year of publication is shown on the last page of each paper.
Frank A. Taylor
Director, United States National Museum
[Pg 177]
Contributions from
The Museum of History and Technology:
Paper 40
History of Phosphorus
Eduard Farber
THE ELEMENT FROM ANIMALS AND PLANTS 178
EARLY USES 181
CHEMICAL CONSTITUTION OF PHOSPHORIC ACIDS 182
PHOSPHATES AS PLANT NUTRIENTS 185
FROM INORGANIC TO ORGANIC PHOSPHATES 187
PHOSPHATIDES AND PHOSPHAGENS 189
NUCLEIN AND NUCLEIC ACIDS 192
PHOSPHATES IN BIOLOGICAL PROCESSES 197
MEDICINES AND POISONS 198
[Pg 178]
Eduard Farber
HISTORY OF PHOSPHORUS
The “cold light” produced by phosphorus caused it to be considered a miraculous chemical for a long time after its discovery, about 1669. During the intervening three centuries numerous other chemical miracles have been found, yet phosphorus retains a special aura of universal importance in chemistry. Many investigators have occupied themselves with this element and its diverse chemical compounds. Further enlightenment and insight into the ways of nature can be expected from these efforts.
Not only is the story of phosphorus a major drama in the history of chemistry; it also illustrates, in a spectacular example, the growth of this science through the discovery of connections between apparently unrelated phenomena, and the continuous interplay between basic science and the search for practical usage.
The Author: Eduard Farber is a research professor at American University, Washington, D.C., and has been associated with the Smithsonian Institution as a consultant in chemistry.
When phosphorus was discovered, nearly three centuries ago, it was considered a miraculous thing. The only event that provoked a similar emotion was the discovery of radium more than two centuries later. The excitement about the Phosphorus igneus, Boyle’s Icy Noctiluca, was slowly replaced by, or converted into, chemical research. Yet, if we would allow room for emotion in research, we could still be excited about the wondrous substance that chemical and biological work continues to reveal as vitally important. It is a fundamental plant nutrient, an essential part in nerve and brain substance, a decisive factor in muscle action and cell growth, and also a component in fast-acting, powerful poisons. The importance of phosphorus was gradually recognized and the means by which this took place are characteristic and similar to other developments in the history of science. This paper was written in order to summarize these various means which led to the highly complex ways of present research.
The Element from Animals and Plants
It was a little late to search for the philosophers’ stone in 1669, yet it was in such a search that phosphorus was discovered. Wilhelm Homberg (1652-1715) described it in the following manner: Brand, [Pg 179]“a man little known, of low birth, with a bizarre and mysterious nature in all he did, found this luminous matter while searching for something else. He was a glassmaker by profession, but he had abandoned it in order to be free for the pursuit of the philosophical stone with which he was engrossed. Having put it into his mind that the secret of the philosophical stone consisted in the preparation of urine, this man worked in all kinds of manners and for a very long time without finding anything. Finally, in the year 1669, after a strong distillation of urine, he found in the recipient a luminant matter that has since been called phosphorus. He showed it to some of his friends, among them Mister Kunkel [sic].”[1]
Neither the name nor the phenomenon were really new. Organic phosphorescent materials were known to Aristotle, and a lithophosphorus was the subject of a book published in 1640, based on a discovery made by a shoemaker, Vicenzo Casciarolo, on a mountain-side near Bologna in 1630.[2] Was the substance new which Brand showed to his friends? Johann Gottfried Leonhardi quotes a book of 1689 in which the author, Kletwich, claims that this phosphorus had already been known to Fernelius, the court physician of King Henri II of France (1154-1189).[3] To the same period belongs the “Ordinatio Alchid Bechil Saraceni philosophi,” in which Ferdinand Hoefer found a distillation of urine with clay and carbonaceous material described, and the resulting product named escarbuncle.[4] It would be worth looking for this source; although Bechil would still remain an entirely unsuccessful predecessor, it does seem strange that in all the distillations of arbitrary mixtures, the conditions should never before 1669 have been right for the formation and the observation of phosphorus.

Figure 1.—The alchemist discovers phosphorus. A painting by Joseph Wright (1734-1779) of Derby, England.
For Brand’s contemporaries at least, the discovery was new and exciting. The philosopher Gottfried Wilhelm von Leibniz (1646-1716) considered it important enough to devote some of his time (between his work as librarian in Hanover and Wolfenbüttel, his efforts to reunite the Protestant and the Catholic churches, and his duties as Privy Councellor in what we would call a Department of Justice) to a history of phosphorus. This friend of Huygens and Boyle tried to prove that Kunckel was not justified in claiming the discovery for himself.[5] Since then, it has been shown that Johann Kunckel (1630-1703) actually worked out the method which neither Brand nor his friend Kraft wanted to disclose. Boyle also developed a method independently, published it, and instructed[Pg 180] Gottfried Hankwitz in the technique. Later on, Jean Hellot (1685-1765) gave a meticulous description of the details and a long survey of the literature.[6]
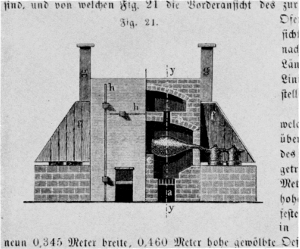
Figure 2.—Galley-oven, 1869. The picture is a cross section through the front of the oven showing one of the 36 retorts, the receivers for the distillate, and the space in the upper story used for evaporating the mixture of acid solution of calcium phosphate and coal. (According to Anselme Payen, Précis de Chimie industrielle, Paris, 1849; reproduced from Hugo Fleck, Die Fabrikation chemischer Produkte aus thierischen Abfällen, Vieweg, Braunschweig, 1862, page 80 of volume 2, 2nd group, of P. Bolley’s Handbuch der chemischen Technologie.)
To obtain phosphorus, a good proportion of coal (regarded as a type of phlogiston) was added to urine, previously thickened by evaporation and preferably after putrefaction, and the mixture was heated to the highest attainable temperature. It was obvious that phlogiston entered into the composition of the distillation product. The question remained whether this product was generated de novo. In his research of 1743 to 1746, Andreas Sigismund Marggraf (1709-1782) provided the answer. He found the new substance in edible plant seeds, and he concluded that it enters the human system through the plant food, to be excreted later in the urine. He did not convince all the chemists with his reasoning. In 1789, Macquer wrote: “There are some who, even at this time, hold that the phosphorical (‘phosphorische’) acid generates itself in the animals and who consider this to be the ‘animalistic acid.’”[7]
Although Marggraf was more advanced in his arguments than these chemists, yet he was a child of his time. The luminescent and combustible, almost wax-like substance impressed him greatly. “My thoughts about the unexpected generation of light and fire out of water, fine earth, and phlogiston I reserve to describe at a later time.” These thoughts went so far as to connect the new marvel with alchemical wonder tales. When Marggraf used the “essential salt of urine,” also called sal microcosmicum, and admixed silver chloride (“horny silver”) to it for the distillation of phosphorus, he expected “a partial conversion of silver by phlogiston and the added fine vitrifiable earth, but no trace of a more noble metal appeared.”[8]
Robert Boyle had already found that the burning of phosphorus produced an acid. He identified it by taste and by its influence on colored plant extracts serving as “indicators.” Hankwitz[9] described methods for obtaining this acid, and Marggraf showed its chemical peculiarities. They did not necessarily establish phosphorus as a new element. To do that was not as important, at that time, as to conjecture on analogies with known substances. Underlying all its unique characteristics was the analogy of phosphorus with sulfur. Like sulfur, phosphorus can burn in two different ways, either slowly or more violently, and form two different acids. The analogy can, therefore, be extended to explain the results in both groups in the same way. In the process of burning, the combustible component is removed, and the acid originally combined with the combustible is set free. Whether the analogy should be pursued even further remained doubtful, although some suspicion lingered on for a while that phosphoric acid might actually be a modified sulfuric acid. Analogies and suspicions like these were needed to formulate new questions and stimulate new experiments. They are cited here for their important positive value in the historical development, and not for the purpose of showing how wrong these chemists were from our[Pg 181] point of view, a point of view which they helped to create.
The widespread interest in the burning of sulfur and of phosphorus, naturally, caught Lavoisier’s attention. In his first volume of Opuscules Physiques et Chimiques (1774), he devoted 20 pages to his experiments on phosphorus. He amplified them a few years later[10] when he attributed the combustion to a combination of phosphorus with the “eminently respirable” part of air. In the Méthode de Nomenclature Chimique of 1787, the column of “undecomposed substances” lists sulfur as the “radical sulfurique,” and phosphorus, correspondingly, as the “radical phosphorique.” The acids are now shown to be compounds of the “undecomposed” radicals, the complete reversion of the previous concept of this relationship. A part of the old analogy remained as far as the acids are concerned: sulfuric acid corresponds to phosphoric; sulfurous acid to phosphorous acid with less oxygen than in the former.[11]
Early Uses
In the 18th century, phosphorus was a costly material. It was produced mostly for display and to satisfy curiosity. Guillaume François Rouelle (1703-1770) demonstrated the process in his lectures, and, as Macquer reports, he “very often” succeeded in making it.[12] Robert Boyle had the idea of using phosphorus as a light for underwater divers.[13] A century later, “instant lights” were sold, with molten phosphorus as the “igniter,” but they proved cumbersome and unreliable.[14] Because white phosphorus is highly poisonous, an active development of the use in matches occurred only after the conversion of the white modification into the red had been studied by Émile Kopp (1844), by Wilhelm Hittorf (1824-1914) and, in its practical application, by Anton Schrötter (1802-1875).[15]
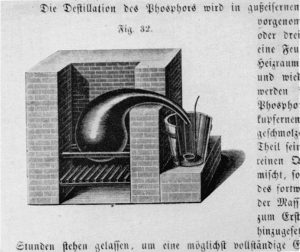
Figure 3.—Distillation apparatus (1849) for refining crude phosphorus. The crude phosphorus is mixed with sand under hot water, cooled, drained, and filled into the retort. The outlet of the retort, at least 6 cm. in diameter, is partially immersed in the water contained in the bucket. A small dish, made from lead, with an iron handle, receives the distilled phosphorus. (From Hugo Fleck, Die Fabrikation chemischer Produkte ... page 90.)
The most exciting early use, however, was in medicine. It is not surprising that such a use was sought at that time. Any new material immediately became the hope of ailing mankind—and of striving inventors.[16] Phosphorus was prescribed, in liniments with fatty oils or as solution in alcohol and ether, for external and internal application. A certain Dr. Kramer found it efficient against epilepsy and melancholia (1730). A Professor Hartmann recommended it against cramps.[17] However, in the growing[Pg 182] production of phosphorus for matches, the workers experienced the poisonous effects. In the plant of Black and Bell at Stratford, this was prevented by inhaling turpentine. Experiments on dogs were carried out to show that poisoning by phosphorus could be remedied through oil of turpentine.[18]
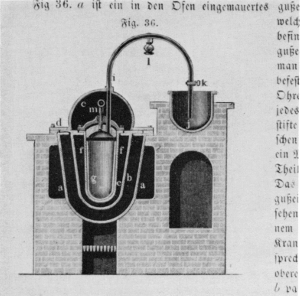
Figure 4.—Apparatus for converting white phosphorus into the red allotropic form, 1851. Redistilled phosphorus is heated in the glass or porcelain vessel (g) which is surrounded by a sandbath (e) and a metal bath (b). Vessel (j) is filled with mercury and water; together with valve (k), it serves as a safety device. The alcohol lamp (l) keeps the tube warm against clogging by solidified vapors. Because of hydrogen phosphides, the operation, carried out at 260° C., had to be watched very carefully. (According to Arthur Albright, 1851; reproduced from Hugo Fleck, Die Fabrikation chemischer Produkte ..., page 112.)
Chemical Constitution of Phosphoric Acids
In a long article on phosphorus, Edmond Willm wrote in 1876: “For a century, urine was the only source from which phosphorus was obtained. After Gahn, in 1769, recognized the presence of phosphoric acid in bones, Scheele indicated the procedure for making phosphorus from them.”[19] Actually, Gahn used at first hartshorn (Cornu cervi ustum), and Scheele doubted, until he checked it himself, that his esteemed friend was right. A few years later, Scheele corrected Gahn’s assumption that the sal microcosmicum was an ammonia salt; instead, it is “a tertiary neutral salt, consisting of alkali minerali fixo (i.e., sodium), alkali volatili, and acido phosphori.”[20]
In the years after 1770, phosphorus was discovered in bones and many other parts of various animals. Treatment with sulfuric acid decomposed these materials into a solid residue and dissolved phosphoric acid. Many salts of this acid were produced in crystalline form. Heat resistance had been considered one of the outstanding characteristics of phosphoric acid. Now, however, in the processes of drying and heating certain phosphates, it became clear that three kinds of phosphoric acids could be produced: ortho, pyro, and meta.
Berzelius cited these acids as examples of compounds which are ISOMERIC. This word was intended to designate compounds which contain the same number of atoms of the same elements but combined in different manners, thereby explaining their different chemical properties and crystal forms. It was in 1830 that Berzelius propounded this companion of the concept, ISOMORPHISM, which was to collect all cases of equal crystal form in compounds in which equal numbers of atoms of different elements are put together in the same manner. Together, the two concepts of isomerism and isomorphism seemed to cover all the known exceptions from the simplest assumption as to specificity and chemical composition.
However, only a few years later Thomas Graham (1805-1869) proved that the three phosphoric acids are not isomeric. He used the proportion of 2 P to 5 O in the oxide which Berzelius had thought justified at least until “an example of the contrary could be sufficiently established.”[21] Refining the techniques of Gay-Lussac (1816) and several other investigators, Graham characterized the three phosphoric acids as “a terphosphate, a biphosphate, and phosphate of water.” Actually, this was the wrong terminology for what he meant and formulated as trihydrate, bihydrate, and monohydrate of phosphorus oxide. In[Pg 183] his manner of writing the formulas, each dot over the symbol for the element was to indicate an atom of oxygen; thus, he wrote:
... :: .. ... . .
H3 P H2 P and H P.[22]

Figure 5.—Oven for the calcination of bones, about 1870. “The operation is carried out in a rather high oven, such as shown.... The fresh bones are thrown in at the top of the oven, B. First, fuel in chamber F is lighted, and a certain quantity of bones is burnt on the grid D. When these bones are burning well, the oven is gradually filled with bones, and the combustion maintains itself without addition of other fuel. A circular gallery, C, surrounds the bottom of the oven and carries the products of combustion into the chimney, H. The calcined bones are taken out at the lower opening, G, by removing the bars of grid B.” (Translation of the description from Figuier, Merveilles de l’industrie, volume 3, 1874, page 537.)

Figure 6.—An advertisement with view of plant for manufacturing superphosphate about 1867. (From E. T. Freedley, Philadelphia and its Manufacturers in 1867, page 288.)
[Pg 184] 
Figure 7.—Florida hard-rock phosphate mining. (From Carroll D. Wright, The Phosphate Industry of the United States, sixth special report of the Commissioner of Labor, Government Printing Office, Washington, 1893, plate facing page 43.)
Graham had come to this understanding of the phosphoric acids through his previous studies of “Alcoates, definite compounds of Salts and Alcohol analogous to the Hydrates” (1831). Liebig started from analogies he saw with certain organic acids when he formulated the phosphoric acids with a constant proportion of water (aq.) and varying proportions of “phosphoric acid” (P) as follows:
[Pg 185]2 P 3 aq. phosphoric acid
3 P 3 aq. pyrophosphoric acid
6 P 3 aq. metaphosphoric acid.
Salts are formed when a “basis,” i.e., a metal oxide, replaces water. When potassium-acid sulfate is neutralized by sodium base, the acid-salt divides into Glauber’s salt and potassium sulfate, which proves the acid-salt to be a mixture of the neutral salt with its acid. Sodium-acid phosphate behaves quite differently. After neutralization by a potassium “base” (hydroxide), the salt does not split up; a uniform sodium-potassium phosphate is obtained. Therefore, phosphoric acid is truly three-basic![23]
This result has later been confirmed, but the analogy by means of which it had been obtained was very weak, in certain parts quite wrong.
The acids from the two lower oxides of phosphorus were also considered as three-basic. Adolphe Wurtz (1817-1884) formulated them in 1846, according to the theory of chemical types:
(PO) · · ·
O3 phosphoric acid
H3
(PHO) · ·
O2 phosphorus acid
H2
(PH2O) ·
O hypophosphorous acid.[24]
H
Further proof for these constitutions was sought in the study of the esters formed when the acids react with alcohols.
Among the analogies and generalizations by which the research on phosphoric acid was supported, and to the results of which it contributed a full share, was the new theory of acids. Not oxygen, Lavoisier’s general acidifier, but reactive hydrogen determines the character of acids. In this brief survey, it seems sufficient just to mention this connection without describing it in detail.
The study of phosphoric acids led to important new concepts in theoretical chemistry. The finding of polybasicity was extended to other acids and formed the model that helped to recognize the polyfunctionality in other compounds, like alcohols and amines. The hydrogen theory of acids was fundamental for further advance. In another dimension, it is particularly interesting to see that large-scale applications followed almost immediately and directly from the new theoretical insight. The first and foremost of these applications was in agriculture.
Phosphates as Plant Nutrients
One hundred years after the discovery of “cold light,” the presence of phosphorus in plants and animals was ascertained, and its form was established as a compound of phosphoric acid. This knowledge had little practical effect until the “nature” of the acid, in its various forms, was explained through the work of Thomas Graham. From it, there started a considerable technical development.
At about that time (1833), the Duke of Richmond proved that the fertilizing value of bones resided not in the gelatin, nor in the calcium, but in the phosphoric acid. Thus, he confirmed what Théodore de Saussure had said in 1804, that “we have no reason to believe” that plants can exist without phosphorus. Unknowingly at first, the farmer had supplied this element by means of the organic fertilizers he used: manure, excrements, bones, and horns. Now, with the value of phosphorus known, a search began for mineral phosphates to be applied as fertilizers. Jean Baptiste Boussingault (1802-1887), an agricultural chemist in Lyons, traveled to Peru to see the guano deposits. Garcilaso de la Vega (ca. 1540 to ca. 1616) noted in his history of Peru (1604) that guano was used by the Incas as a fertilizer. Two hundred years later, Alexander von Humboldt revived this knowledge, and Humphry Davy wrote about the benefits of guano to the soil. Yet, the application of this fertilizer developed only slowly, until Justus Liebig sang its praise. Imports into England rose and far exceeded those into France where, between 1857 and 1867, about 50,000 tons were annually received.
The other great advance in the use of phosphatic plant nutrients started with Liebig’s recommendation (1840) to treat bones with sulfuric acid for solubilization. This idea was not entirely new; since 1832, a production of a “superphosphate” from bones and sulfuric acid had been in progress at Prague. At[Pg 186] Rothamsted in 1842, John Bennet Lawes obtained a patent on the manufacture of superphosphate. Other manufactures in England followed and were successful, although James Muspratt (1793-1886) at Newton lost much time and “some thousands of pounds” on Liebig’s idea of a “mineral manure.”

Figure 8.—Florida land-pebble phosphate mining. (From Carroll D. Wright, The Phosphate Industry of the United States ..., plate facing page 58.)
It was difficult enough to establish the efficacy of bones and artificially produced phosphates in promoting the growth of plants under special conditions of soils and climate; therefore, the question as to the action of phosphates in the growing plant was not even seriously formulated at that time. The beneficial effects were obvious enough to increase the use of phosphates as plant nutrients and to call for new sources of supply. Active developments of phosphate mining and treating started in South Carolina in 1867, and in Florida in 1888.[25]
In a reciprocal action, more phosphate application to soils stimulated increasing research on the conditions and reactions obtaining in the complex and varying compositions called soil. The findings of bacteriologists made it clear that physics and chemistry had to be amplified by biology for a real understanding of fertilizer effects. After 1900, for example, Julius Stoklasa (1857-1936) pointed out that bacterial action in soil solubilizes water-insoluble phosphates and makes them available to the plants.[26]

Figure 9.—Florida river-pebble phosphate mining. (From Carroll D. Wright, The Phosphate Industry of the United States ..., plate facing page 64.)
The insight into the importance of phosphorus in organisms, especially since Liebig’s time, is reflected in the work of Friedrich Nietzsche (1844-1900). This “re-valuator of all values” who modestly said of himself: “I am dynamite!” once explained the human temperaments as caused by the inorganic salts they contain: [Pg 187]“The differences in temperament are perhaps caused more by the different distribution and quantities of the inorganic salts than by everything else. Bilious people have too little sodium sulfate, the melancholics are lacking in potassium sulfate and phosphate; too little calcium phosphate in the phlegmatics. Courageous natures have an excess of iron phosphate.” (See volume 12 of Nietzsche’s Works, edit. Naumann-Kröner, Leipzig, 1886.) In this strange association of inorganic salts with human temperaments, the role of iron phosphate as a producer of courage is particularly interesting. What would a modern philosopher conclude if he followed the development of insight into the composition and function of complex phosphate compounds in organisms?
From Inorganic to Organic Phosphates
By the middle of the 19th century, the source of phosphorus in natural phosphates and the chemistry of its oxidation products had been established. The main difficulty that had to be overcome was that these oxidation products existed in so many forms, not only several stages of oxidation, but, in addition, aggregations and condensations of the phosphoric acids. Once the fundamental chemistry of these acids was elucidated, the attention of chemists and physiologists turned to the task of finding the actual state in which phosphorus compounds were present in the organisms. It had been a great advance when it had been shown that plants need phosphates in their soil. This led to the next question concerning the materials in the body of the plant for which phosphates were being used and into which they were incorporated. Similarly, the knowledge that animals attain their phosphates from the digested plant food called, in the next step of scientific inquiry, for information on the nature of phosphates produced from this source.
The method used in this inquiry was to subject anatomically separated parts of the organisms to chemical separations. The means for such separations had to be more gentle than the strong heat and destructive chemicals that had been considered adequate up to then. The interpretation of the new results naturally relied on the general advance of chemistry, the development of new methods for isolating substances of little stability, of new concepts concerning the arrangements of atoms in the molecules, and of new apparatus to measure their rates of change.[Pg 188]
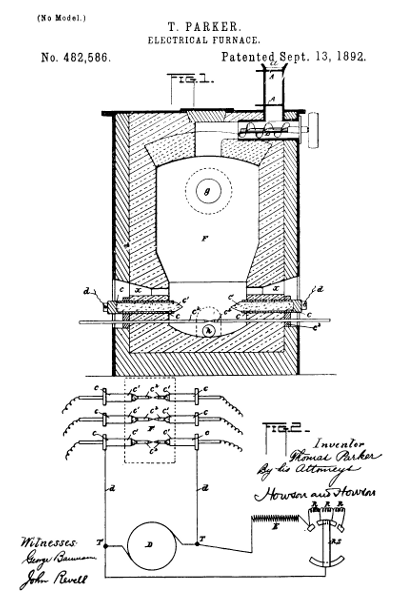
Figure 10.—Electric furnace for producing elemental phosphorus, invented by Thomas Parker of Newbridge, England, and assigned to The Electric Construction Corporation of the same place. The drawing is part of United States patent 482,586 (September 13, 1892). The furnace was patented in England on October 29, 1889 (no. 17,060); in France on June 23, 1890 (no. 206,566); in Germany on June 17, 1890 (no. 55,700); and in Italy on October 23, 1890 (no. 431). The following explanation is cited from the U.S. patent:
Figure 1 [shown here] is a vertical section of the furnace, and Fig. 2 is a diagram to illustrate the means for regulating the electro-motive force or quantity of current across the furnace.
F is the furnace containing the charge to be treated. It has an inlet-hopper at a, with slides AA, by which the charge can be admitted without opening communication between the interior of the furnace and the outer air.
B is a screw conveyer by which the charge is pushed forward into the furnace.
c´c´ are the electrodes, consisting of blocks or cylinders or the like of carbon fixed in metal socket-pieces c c, to which the electric-circuit wires d from the dynamo D are affixed. The current, as aforesaid, may be either continuous or alternating. c2c2 are rods of metal or carbon, which are used to establish the electric circuit through the furnace, the said rods being inserted into holes in conductors c3 (in contact with the socket-pieces c) and in the furnace, as shown.
g is the outlet for the gas or vapor, h the slag-tap hole, and x the opening for manipulating the charge, the said openings being closed by clay or otherwise when the furnace is at work.
I use coke or other form of carbon in the charge between the electrodes c´, the said coke being in contact with the said electrodes, so that complete incandescence is insured.
A means for varying the electro-motive force or quantity of current across the furnace with the varying resistance of the charge is illustrated by the diagram, Fig. 2. c´ c2 indicate the electrodes in the furnace, as in Fig. 1, and D is the dynamo and T its terminals. E represents the exciting-circuit. R R are resistances, and R S is the resistance-switch, which is operated to put in more or less resistance at R as the resistance of the charge in the furnace lessens or increases. This switch may be automatically operated, and a suitable arrangement for the purpose is a current-regulator such as is described in the specification of English Letters Patent No. 14,504, of September 14, 1889, granted to William Henry Douglas and Thomas Hugh Parker.
[Pg 189]

Figure 11.—Dipping of matchsticks in France, about 1870. The frame which holds the matches so that one end protrudes at the bottom, is lowered over a pan containing molten sulfur. The sulfur-covered matches are then dropped into a phosphorous paste. See figure 12. (From Figuier, Merveilles de l’industrie, volume 3, 1874, page 575.)
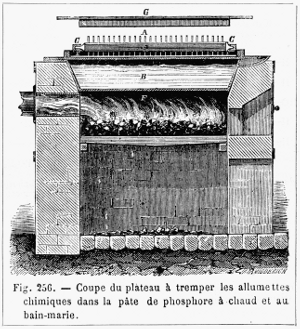
Figure 12.—Pan for dipping matchsticks into phosphorus paste, about 1870. The letters on the picture are: A, matches; B, water bath; C, frame; D, plate; E, phosphorus paste; F, oven. The phosphorus paste of Böttger, 1842, contained 10 phosphorus, 25 antimony sulfide, 12.5 manganese dioxide, 15 gelatin. According to Figuier (page 579), R. Wagner substituted lead dioxide for the manganese dioxide. (From Figuier, volume 3, 1874, page 576.)
In the system of chemistry, as it developed in the first half of the 19th century, the new development can be characterized as the turn from inorganic to organic phosphates, from the substance of minerals and strong chemical interactions to the components in which phosphate groups remained combined with carbon-containing substances.
Phosphatides and Phosphagens
The important phosphorus compounds in organisms are much more complex than the simple salts, to which Nietzsche attributed such influence on man’s character. Long before he wrote, it was known that phosphoric acid combines not only with inorganic bases to form salts, but with alcohols to form esters. In the middle of the 19th century, Théophile Juste Pelouze (1807-1867) extended this knowledge to an ester of glycerol. This proved to be significant in several respects. Glycerol had been shown by Michel Chevreul (1786-1889) as the substance in fats that is released in the process of soap boiling, when the fatty acids are converted into their salts. That it has the nature of an alcohol had been demonstrated by Marcellin Berthelot. Instead of one “alcoholic” hydroxyl group, OH, like ethanol (the alcohol of fermentation), or two hydroxyl groups (like ethylene glycol), glycerol contains three such groups. It was the only “natural” alcohol known at that time. That this alcohol would combine with phosphoric acid could be predicted, but that the ester, as obtained by Pelouze, still contained free acidic functions and formed a water-soluble barium salt was a new experience.
[Pg 190]
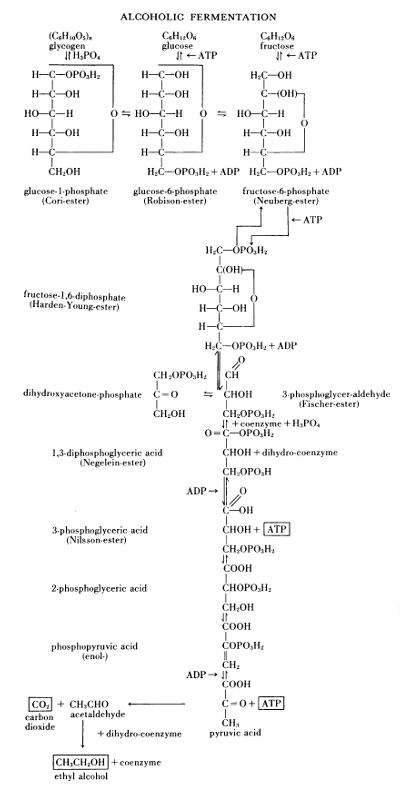
Figure 13.—Survey of alcoholic fermentation, 1951. The “well-known scheme of alcoholic fermentation” according to Albert Jan Kluyver (1888-1956), presented before the Society of Chemical Industry in the Royal Institution, March 7, 1951. In Chemistry & Industry, 1952, page 136 ff., Kluyver restates that “... the fermentation of one molecule of glucose is indissolubly connected with the formation of two molecules of adenosine triphosphate (ATP) out of two molecules of adenosine diphosphate (ADP).”
[Pg 191] Shortly after this experience had been gained, it became valuable for understanding the chemical nature of a new substance extracted from a natural organ. This substance was named lecithin by its discoverer, Nicolas Théodore Gobley[27] (1811-1876), because he obtained it from egg yolk (in Greek, lékidos). He used ether and alcohol for this extraction. Had he used water and mineral acid instead, he would not have found lecithin, but only its components. As Gobley and, slightly later, Oscar Liebreich (1839-1908), subjected lecithin to treatment with boiling water and acid, they separated it into three parts. One of them was the glycerophosphoric acid of Pelouze, the second was the well-known stearic acid of Chevreul, but the third was somewhat mysterious. This third substance was the same as one previously noticed when nerves had been subjected to an extraction by boiling water and acid and, therefore, called nerve-substance or neurine. Adolf Friedrich Strecker (1822-1871) established the identity of this neurine with a product he had extracted from bile and which went under the name of choline. Adolphe Wurtz (1817-1884) succeeded in synthesizing this substance from ethylene oxide, CH2.O.CH2 and trimethylamine N(CH3)3.[28] Thus, all three parts were identified, and Strecker put them together to construct a chemical formula for lecithin, glycerophosphoric acid combined with a fatty acid and with choline (a hydrate of neurine).
|
{ |
OH |
} |
|
| N |
(CH3)3 |
Choline |
|
C2H4O |
|
| |
| C18H33O2 |
} |
HO |
} |
|
|
PO |
| C16H31O2 |
C3H5O |
| Fatty Acids |
|
Glycerophosphate |
| '—————v————' |
| Lecithin |
| according to Strecker |
This formula was not quite correct. Richard Willstätter showed that an internal neutralization takes place between the amino group and the free acidic residue. This is expressed in his lecithin formula of 1918.
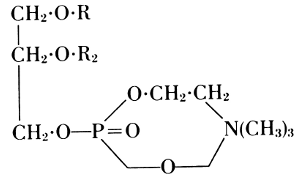
Lecithin (1918)
When the aim was to distill elementary phosphorus out of an organic material, it did not matter whether this was fresh or putrified. For obtaining lecithin out of egg yolk and similar materials, it was essential to use it in fresh condition. Otherwise, enzymes would have decomposed it. Through more recent work, four enzymes have been separated, which act specifically in decomposing lecithin. Enzyme A removes one fatty acid and leaves a complex residue, called lysolecithin, intact. Enzyme B attacks this residue and splits off the remaining fatty acid group from it, enzyme C liberates only the choline from lecithin, and enzyme D opens lecithin at the ester bond between glycerol and phosphoric acid. This is shown in the following diagram.
| Enzymatic Splitting of Lecithins |
| Enzyme |
Substrate |
Products |
| A |
Lecithin |
Lysolecithin and fatty acids. |
| B |
Lysolecithin |
Glycero-phospho-choline and fatty acids. |
| C |
Lecithin |
Phosphatidic acid and choline. |
| D |
Lecithin |
Phosphoryl choline and diglyceride. |
Several fatty acids can be present in lecithin from various sources: palmitic and oleic acid, besides the stearic acid which at first had been thought the only one involved. In another group of extracts from brain or nerve tissue, amino-ethanol H2NCH2CH2OH is found instead of the choline of lecithin. The variations include the alcohol, to which the fatty acids and choline phosphate are attached, for example, glycerol can be replaced by the so-called meat-sugar, inositol, which has six hydroxyl groups in its hexagon-shaped molecule [Pg 192]C6H6(OH)6.

Figure 14.—Eduard Buchner (1860-1917) received the Nobel Prize in Chemistry for his discovery of cell-free fermentation, the first step in finding the role of phosphate in fermentations (1907).
The generally similar behavior of these phosphate-and fat-containing substances was emphasized by Ludwig Thudichum (1829-1901). He coined the name phosphatides for this group of substances from seeds and nerves.[29] His work on the phosphates in brain substance aroused particular interest. When William Crookes drew his highly imaginative picture of an “evolution” of the chemical elements, he put into it “phosphorus for the brain, salt for the sea, clay for the solid earth....”[30] But phosphatides occur in many places of organisms, in bacteria, in leaves and roots of plants, in fat and tissues of animals. And where phosphatides are found, there are also enzymes that specifically act on them. They are called phosphatases to imply that they split the phosphatides. In addition, enzymes are present, which transfer phosphate groups from one compound to another. They are more abundant in seeds of high fat content than in the more starch-containing seeds, but even potatoes and orange juice have phosphatases.[31]
Thus, from phosphatides, phosphoric acid is generated, and they could also be called phosphagens. Since 1926, however, the name phosphagens has been reserved for a group of organic substances that release their phosphoric acid very readily. The link between phosphorus and carbon is provided by oxygen in the phosphatides, by nitrogen in the phosphagens. In vertebrates, the basis for the phosphoric acid is creatine, whereas invertebrates have arginine instead.
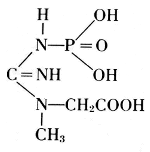
Creatine Phosphate
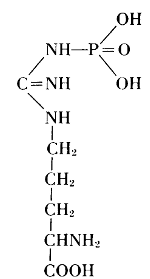
Arginine phosphate
Nuclein and Nucleic Acids
All parts of an organism are essential for life. Only with this in mind does it make sense to say that the most important part of the cell is its nucleus. From the nuclei of cells in pus and in salmon sperm, Johann Friedrich Miescher (1811-1887) obtained a peculiar kind of substance, which he named nuclein (1868). Its phosphate content was easily discovered, but to find the exact proportions and the nature of the other components required special methods of separation from phosphatides and other proteins. It was difficult to develop such methods at a time when little was known about the properties, and particularly[Pg 193] the stability, of a nuclein. For preparing nuclein from yeast cells, Felix Hoppe-Seyler (1825-1895) described the following details: Yeast is dispersed in water to extract soluble materials, like salts or sugars. After a few hours, the insoluble material is separated, washed once more with water, and then extracted with a very dilute solution of sodium hydroxide. The slightly alkaline solution, freed from insoluble residues, is slowly added to a weak hydrochloric acid. A precipitate forms which is separated by filtration, washed with dilute acid, then with cold alcohol, and finally extracted by boiling alcohol. The dried residue is the nuclein.[32] It contains six percent phosphorus. A little more washing with water, a slightly longer treatment with acid or alcohol gives products of lower phosphorus content. Many experimental variations were necessary to establish the procedure that leads to purification without alteration of the natural substance.
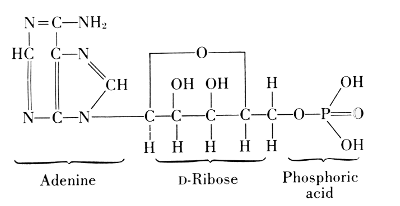
This was also true for the methods of chemical degradation, carried out in order to find the components of nucleins in their highest state of natural complexity. It was learned for example, that the special kind of carbohydrate present in nucleins was very susceptible to change under the conditions of hydrolysis by acids. Phoebus Aaron Theodor Levine (1869-1940), therefore, used the digestion by a living organism. With E. S. London, he introduced a solution of nucleic acid into, e.g., the gastrointestinal segment of a dog through a gastric fistula and withdrew the product of digestion through an intestinal fistula. Fortunately, the products obtained in such degradations were not new in themselves. The carbohydrate in this nucleic acid proved to be identical with D-ribose, which Emil Fischer had artificially made from arabinose and named ribose to indicate this relationship (1891). The nitrogenous products of the degradation were identical with substances previously prepared in the long study of uric acid. In the course of this study, Emil Fischer established uric acid and a number of its derivatives as having the elementary skeleton of what he called “pure uric acid,” abbreviated to purine. Out of Adolf Baeyer’s work on barbituric acid came the knowledge of pyrimidine and its derivatives.

Figure 15.—Albrecht Kossel (1853-1927) received the Nobel Prize in Medicine and Physiology in 1910 for his work on nucleic substances, which contain a high proportion of phosphorus. The chemical bonds of this phosphorus in the molecules of nucleic substances were determined in later work. (Photo courtesy National Library of Medicine, Washington, D.C.)
From these findings, together with what Oswald Schmiedeberg (1838-1921) had established concerning the presence of four phosphate groups in the molecule (1899), Robert Feulgen (1884-1955) constructed the following scheme of a nucleic acid. Feulgen’s formula of 1918 is:
Phosphoric acid—Carbohydrate—Guanine
Phosphoric acid—Carbohydrate—Cytosine
Phosphoric acid—Carbohydrate—Thymine
Phosphoric acid—Carbohydrate—Adenine
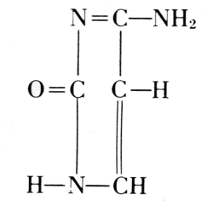
Of the four basic components on the right, thymine occurs in the nucleic acid from the thymus gland. Yeast contains uracil instead. The difference between these two bases is one methyl group: thymine is a 5-methyluracil. In all of these basic substances, the structure of urea
NH2
/
C=O
\
NH2
is involved, and they form pairs of oxidized and reduced states: [Pg 194]
| Purine |
Pyrimidine |
| (reduced) Adenine |
+ |
(oxidized) Thymine |
| (oxidized) Guanine |
+ |
(reduced) Cytosine |
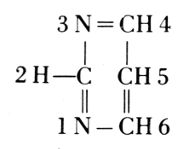
Pyrimidine
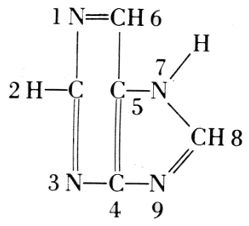
Purine
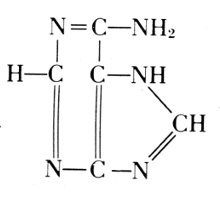
Adenine
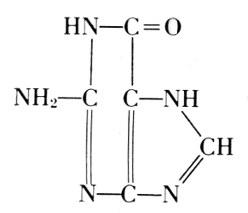
Guanine
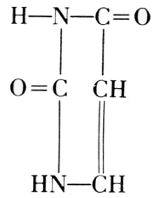
Uracil
Cytosine
The carbohydrate is ribose or deoxyribose.
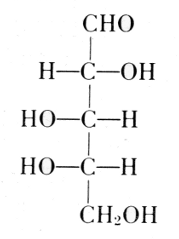
Arabinose
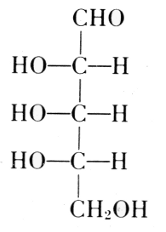
l-Ribose
Fischer and Piloty, 1891
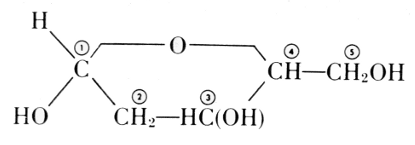
Deoxyribose
The exact position of phosphoric acid was established after long work and verified by synthesis.[33]
A compound of adenine, ribose, and phosphoric acid was found in yeast, blood, and in skeletal muscle of mammals. From 100 grams of such muscle, 0.35-0.40 grams of this compound were isolated. If the muscle is at rest, the compound contains three molecules of phosphoric acid, linked through oxygen atoms. It was named adenosine triphosphate or adenyltriphosphoric acid,[34] usually abbreviated by the symbol ATP. It releases one phosphoric acid group very easily and goes over in the diphosphate, ADP, but it can also lose 2 P-groups as pyrophosphoric acid and leave the monophosphate, AMP.
This change of ATP was considered to be the main source of energy in muscle contraction by Otto Meyerhof.[35] The corresponding derivatives of guanine, cytosine, and uracil were also found, and they are active in the temporary transfer of phosphoric acid groups in biological processes.
Thus, the study of organic phosphates progressed from the comparatively simple esters connected with fatty substances of organisms to the proteins and the nuclear substances of the cell. The proportional amount of phosphorus in the former was larger than in the latter; the actual importance and function in the life of organisms, however, is not measured by the quantity but determined by the special nature of the compounds.[Pg 195]

Figure 16.—Otto Meyerhof (1884-1951) received one-half of the Nobel Prize in Medicine and Physiology in 1922 for his discovery of the metabolism of lactic acid in muscle, which involves the action of phosphates, especially adenosine duophosphates. (Photo courtesy National Library of Medicine, Washington, D.C.)
Figure 17.—Arthur Harden (1865-1940), left, and Hans A. S. von Euler-Chelpin (b. 1875), right, shared the Nobel Prize in Chemistry in 1929. Harden received it for his research in fermentation, which showed the influence of phosphate, particularly the formation of a hexose diphosphate. Euler-Chelpin received his award for his research in fermentation. He found coenzyme A which is a nucleotide containing phosphoric acid.
[Pg 196]

Figure 18.—George de Hevesy (b. 1885) received the Nobel Prize in Chemistry in 1943 for his research with isotopic tracer elements, particularly radiophosphorus of weight 32 (ordinary phosphorus is 31).
Figure 19.—Carl F. Cori (b. 1896) and his wife, Gerty T. Cori (1896-1957) received part of the Nobel Prize in Medicine and Physiology in 1947 for their study on glycogen conversion. In the course of this study, they identified glucose 1-phosphate, now usually referred to as “Cori ester,” and its function in the glycogen cycle. (Photo courtesy National Library of Medicine, Washington, D.C.)
[Pg 197] The study of this function is the newest phase in the history of phosphorus and represents the culmination of the previous efforts. This newest phase developed out of an accidental discovery concerning one of the oldest organic-chemical industries, the production of alcohol by the fermentative action of yeast on sugar. A transition of carbohydrates through phosphate compounds to the end products of the fermentation process was found, and it gradually proved to be a kind of model for a host of biological processes.
Specific phosphates were thus found to be indispensable for life. In reverse, the wrong kind of phosphates can destroy life. As a result, an important part of the new phase in phosphorus history consisted in the study—and use—of antibiotic phosphorus compounds.
Phosphates in Biological Processes
The first indication that phosphorus is important for life came from the experience that plants take it up from the substances in the soil. They incorporate it in their body substance. What makes phosphorus so important that they cannot grow without it? The next insight was that animals acquire it from their plant food. It is then found in bones, in fat and nerve tissue, in all cells and particularly in the cell nuclei. What are its functions there?
The answers to such questions were developed from the study of a long-known process, the conversion of carbohydrates into carbon dioxide and alcohol by yeast. It started with Eduard Buchner’s discovery of 1890, that fermentation is produced by a preparation from yeast in which all living cells have been removed. When yeast is dead-ground and pressed out, the juice still has the ability to produce fermentation.
It is strange, but in many ways characteristic for the process of science, that the “riddle” of phosphorus in life was solved by first eliminating life. In such “lifeless” fermentations, Arthur Harden found that the conversion of sugar begins with the formation of a hexose phosphate (1904). The “ferment” of yeast, called zymase, proved to be a composite of several enzymes. Hans von Euler-Chelpin isolated one part of zymase, which remains active even after heating its solution to the boiling point. From 1 kilogram of yeast, he obtained 20 milligrams of this heat-stable enzyme, which he called cozymase and identified as a nucleotide composed of a purine, a sugar, and phosphoric acid.[36] In the years between the two World Wars, zymase was further resolved into more enzymes, one of them the coenzyme I, which was shown to be ADP connected with another molecule of ribose attached to the amide of nicotinic acid, or diphosphopyridine nucleotide:
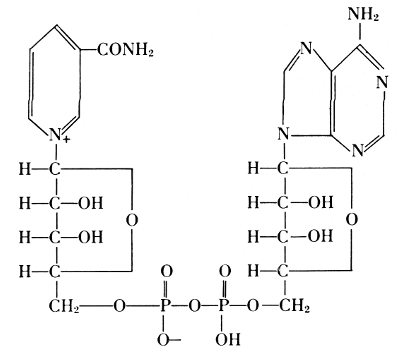

Figure 20.—Fritz A. Lipmann (b. 1899) shared with Hans Adolf Krebs the Nobel Prize in Medicine and Physiology in 1953 for his work on coenzyme A. He discovered acetyl phosphate as the substance in bacteria, which transfers phosphate to adenylic acid.
[Pg 198]

Figure 21.—Alexander R. Todd (b. 1907) received the Nobel Prize in Chemistry in 1957 for his research on nucleotides. He determined the position of the phosphate groups in the molecule and confirmed it by synthesis of dinucleotide phosphates.
Its function is connected with the transfer of hydrogen between intermediates formed through phosphate-transferring enzymes. Fermentation proceeds by a cascade of processes, in which phosphate groups swing back and forth, and equilibria between ATP with ADP play a major role.
Many of the enzymes are closely related to vitamins. Thus, cocarboxylase A, which takes part in the separation of carbon dioxide from an intermediate fermentation product, is the phosphate of vitamin B1. Others of the B vitamins contain phosphate groups, for example those of the B2 and B6 group, and in B12, one lonely phosphate forms a bridge in the large molecule that contains one atom of cobalt: C63H90N14O14PCo. The formation of vitamin A from carotine occurs under the influence of ATP.
The first stages in fermentation are like those in respiration, which ends with carbon dioxide and water. These two are the materials for the reverse process in photosynthesis. When light is absorbed by the chlorophyll of green plants, one of the initial reactions is a transfer of hydrogen from water to a triphosphopyridine nucleotide, which later acts to reduce the carbon dioxide. Under the influence of ATP, phosphoglyceric acid is synthesized and further built up by way of carbohydrate phosphates to hexose sugars and finally to starch. In many starchy fruits, a small proportion of phosphate remains attached to the end product.
The synthesis of proteins is under the control of deoxyribonucleic acid or ribonucleic acid, abbreviated by the symbols DNA and RNA. The genes in the nucleus are parts of a giant DNA molecule. RNA is a universal constituent of all living cells. Where protein synthesis is intense, the content in RNA is high. Thus, the spinning glands of silkworms are extraordinarily rich in RNA.[37]
In his research on the radioactive isotope P32, George de Hevesy gained some insight into the surprising mobility of phosphates in organisms: “A phosphate radical taken up with the food may first participate in the phosphorylation of glucose in the intestinal mucose, soon afterwards pass into the circulation as free phosphate, enter a red corpuscle, become incorporated with an adenosine triphosphoric-acid molecule, participate in a glycolytic process going on in the corpuscle, return to circulation, penetrate into the liver cells, participate in the formation of a phosphatide molecule, after a short interval enter the circulation in this form, penetrate into the spleen, and leave this organ after some time as a constituent of a lymphocyte. We may meet the phosphate radical again as a constituent of the plasma, from which it may find its way into the skeleton.”[38] Much has been added in the last 30 years to complete this picture in many details and to extend it to other biochemical processes, including even the changes of the pigments in the retina in the visual process, or in the conversion of chemical energy to light by bacteria and insects.
Medicines and Poisons
In the delicate balance of these processes, disturbances may occur which can be remedied by specific phosphate-containing medicines. Thus, adenosine phosphate has been recommended in cases of angina[Pg 199] pectoris and marketed under trade names like sarkolyt, or in compounds named angiolysine. A considerable number of physiologically active organic phosphates can be found in the patent literature.[39] Yeast itself is considered to be a valuable food additive.
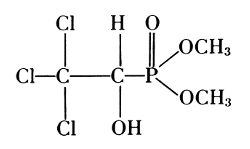
On the other hand, there are phosphate compounds that act as poisons. One group of such compounds was discovered in 1929 by W. Lange, who wrote: “Of interest is the strong action of mono-fluorophosphate esters on the human body—the effect is produced by very small quantities.”[40] Diisopropyl fluorophosphate has since become a potential agent for chemical warfare. It inactivates an enzyme which controls the transmission of nerve impulses to muscle, acetylcholine esterase.
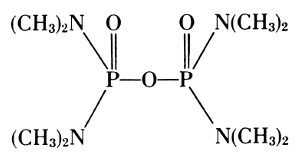
Organic esters of phosphoric acids are used as insecticides. The hexa-ethylester of tetraphosphoric acid, prepared by Gerhard Schrader by heating triethylphosphate with phosphorus oxychloride,[41] actually contains tetraethylpyrophosphate (TEPP) among others. Bayer’s Dipterex, the dimethyl ester of 2,2,2-trichloro-1-hydroxyethyl-phosphonate, has been modified to dimethyl-2,2-dichlorovinyl-phosphate and is especially active against the oriental fruit fly.[42]
Bayer's L 13/59
(Dipterex)
Schradan
Octamethylpyrophosphoramide
Figure 22.—Arthur Kornberg (b. 1918) and Severo Ochoa (b. 1905) shared the Nobel Prize in Medicine and Physiology in 1959. Kornberg received it for research on the biological synthesis of deoxyribonucleic acid. In particular, he found that four triphosphate components and a small amount of the end product as a “template” had to be present for the enzymatic synthesis. Ochoa received his share of the prize for research in ribonucleic acid and deoxyribonucleic acid. In particular, Ochoa synthesized polyribonucleotides and used the radioactive isotope, P32. The synthetic polyribonucleotides were found to resemble the natural substances in all essentials.

Figure 23.—Melvin Calvin (b. 1911) received the Nobel Prize in Chemistry in 1961 for his research in photosynthesis, in which he specified the function of phosphoglyceric acid as an intermediate in the synthesis of carbohydrates from carbon dioxide and water by green plants.
The story of phosphorus, which began 300 years ago, has acquired new importance in this century. Many scientists have contributed to it: 13 of them have received Nobel Prizes for work directly bearing on the chemical and biological importance of phosphorus compounds. In chronological order, they are: Eduard Buchner, Albrecht Kossel, Otto Meyerhof, Arthur Harden, Hans von Euler-Chelpin, George de Hevesy, Carl F. Cori, Gerty T. Cori, Fritz Lipmann, Lord Alexander Todd, Arthur Kornberg, Severo Ochoa, and Melvin Calvin. The developers of industrial production and commercial utilization of phosphate compounds have had other rewards.
Some impression of the continuing growth in this field[43] can be gained from the following data.
Phosphate Rock
annually “sold or used by producer” in the United States in million long tons (2,240 lbs.)
| 1880 |
0.2 |
| 1890 |
0.5 |
| 1900 |
1.5 |
| 1910 |
2.655 |
| 1920 |
4.104 |
| 1930 |
3.926 |
| 1940 |
4.003 |
| 1945 |
5.807 |
| 1950 |
11.114 |
| 1955 |
12.265 |
| 1955 |
(world: about 56) |
| 1960 |
17.202 |
| 1962 |
19.060 |
Sources: U.S. Bureau of the Census. Historical Statistics of the United States 1789-1945 (1949); Statistical Abstract of the United States.
Elemental Phosphorus
annually produced in the United States in short tons (2,000 lbs.)
| 1939 |
43,000 |
| 1944 |
85,679 |
| 1950 |
153,233 |
| 1956 |
312,200 |
| 1958 |
335,750 |
| 1959 |
366,350 |
| 1960 |
409,096 |
| 1961 |
430,617 |
| 1962 |
451,970 |
Source: U.S. Department of Commerce.
FOOTNOTES:
[1] Wilhelm Homberg, Mémoires Académie, 1666-1699 (Paris, 1730), vol. 10, under date of April 30, 1692, pp. 57-61.
[2] Fortunio Licetus, Lithiophosphorus sive de lapide Bononiensi (Venice, 1640).
[3] Cited in Peter Joseph Macquer Chymisches Wörterbuch, 2nd ed. (Leipzig: Weidmann, 1789), vol. 4, p. 508, footnote “c” as “Kletwich (de phosph. liqu. et solid. 1689, Thes. II).”
[4] Ferdinand Hoefer, Histoire de la Chimie (Paris, 1843), vol. 1, p. 339.
[5] G. W. von Leibniz, Mémoires Académie (Paris, 1682); Akademie der Wissenschaften, Miscellanea Berolinensia (Berlin, 1710), vol. 1, p. 91.
[6] Jean Hellot, Mémoires Académie 1737 (Paris, 1766), under date of November 13, 1737, pp. 342-378.
[7] Macquer, op. cit. (footnote 3), p. 551.
[8] A. S. Marggraf, Akademie der Wissenschaften, Miscellanea Berolinensia (Berlin, 1743), vol. 7, 342 ff.; see also Wilhelm Ostwald Klassiker der Exakten Naturwissenschaften (Leipzig: Engelmann, 1913), no. 187.
[9] G. Hanckewitz, [Hankwitz], Philosophical Transactions of the Royal Society of London, 1724-1734, abridged (London, 1809), vol. 7, pp. 596-602.
[10] Antoine Laurent Lavoisier, “Sur la Combustion du Phosphore de Kunckel, Et sur la nature de l’acide qui resulte de cette Combustion,” Mémoires Académie 1777, (Paris, 1780), pp. 65-78.
[11] Guyton de Morveau and others, Méthode de Nomenclature Chimique, Proposée par MM. de Morveau, Lavoisier, Bertholet, & de Fourcroy (Paris, 1787), plate 9.
[12] Macquer, op. cit. (footnote 3), p. 513.
[13] Marie Boas, Robert Boyle and Seventeenth Century Chemistry (New York: Cambridge University Press, 1958), p. 226; see also Wyndham Miles, “The History of Dr. Brand’s Phosphorus Elementarus,” Armed Forces Chemical Journal (November-December 1958), p. 25.
[14] Archibald Clow and Nan L. Clow, The Chemical Revolution (London: Batchworth Press, 1952), p. 451.
[15] Émile Kopp, Comptes-rendus hebdomadaires des Séances de l’Académie des Sciences, Paris (1844), vol. 18, p. 871; Wilhelm Hittorf, Annalen der Chemie und Pharmazie, suppl. to vol. 4, p. 37; Anton Schrötter, Annales de Chimie et de Physique, series 3, vol. 24 (1848), p. 406; see also Schrötter’s report on “Phosphor und Zündwaaren” in A. W. von Hofmann, Bericht über die Entwicklung der Chemischen Industrie (Braunschweig: Vieweg, 1875), pp. 219-246.
[16] R. Glauber, Furni Novi Philosphici (Amsterdam, 1649), vol. 2, pp. 12 ff.
[17] Hermann Schelenz, Geschichte der Pharmazie (Berlin: Springer, 1904), p. 598.
[18] J. Personne, Comptes-rendus ..., Paris (1869), vol. 68, pp. 543-546.
[19] A. Wurtz, Dictionnaire de Chimie (Paris, 1876), vol. 2, part 2, p. 951.
[20] Karl W. Scheele, Nachgelassene Briefe und Aufzeichnungen, edit. A. E. Nordenskiöld (Stockholm: Norstedt, 1892), pp. 38, 144.
[21] J. J. Berzelius, Lehrbuch, transl. F. Wöhler (Dresden, 1827), vol. 3, part 1, p. 96.
[22] Thomas Graham, Philosophical Transactions of the Royal Society of London (1833), pp. 253-284.
[23] Justus Liebig’s Annalen der Pharmacie (1838), vol. 26, p. 113 ff.
[24] A. Wurtz, Annales de Chimie et de Physique, series 3, vol. 16 (1846), p. 190.
[25] Carroll D. Wright, The Phosphate Industry in the United States, sixth special report of the Commissioner of Labor (Washington, 1893).
[26] J. Stoklasa, Biochemischer Kreislauf des Phosphat-Ions im Boden, Centralblatt für Bakteriologie ... (Jena: Fischer, March 22, 1911), vol. 29, nos. 15-19.
[27] N. T. Gobley, Comptes-rendus ..., Paris (1845), vol. 21, p. 718.
[28] A. Wurtz, Comptes-rendus ..., Paris (1868), vol. 66, p. 772.
[29] L. Thudichum, Die chemische Constitution des Gehirns des Menschen und der Tiere (1901); see also H. Wittcoff, The Phosphatides (New York: Reinhold, 1951).
[30] William Crookes, British Association for the Advancement of Science, Reports (1887), sec. B, p. 573.
[31] J. E. Courtois and A. Lino, Progress in the Chemistry of Organic Natural Products, edit. L. Zechmeister (Vienna: Springer Verlag, 1961), vol. 19, p. 316-373.
[32] A. Wurt, Dictionnaire de Chimie, supp. part 2, [n.d.] p. 1087; A. Kossel, Zeitschrift für physiologische Chemie, series 3 (1879), p. 284.
[33] Alexander Todd, Les Prix Nobel en 1957 (Stockholm).
[34] Hans von Euler-Chelpin, Les Prix Nobel en 1929 (Stockholm).
[35] O. Meyerhof and E. Lundsgaard, Naturwissenschaften (Berlin, 1930), vol. 18, pp. 330, 787.
[36] K. Lohmann, Naturwissenschaften (Berlin, 1929), vol. 17, p. 624; C. H. Fiske and Y. Subbarow, Science (Washington, 1929), vol. 70, p. 381 f.
[37] J. Brachet, Scientia, Revista di Scienza (1960), vol. 95, p. 119.
[38] George de Hevesy, Les Prix Nobel en 1940 (Stockholm). See also Eduard Farber, Nobel Prize Winners in Chemistry, 2nd ed. (New York: Schuman, 1963), p. 179.
[39] See, e.g., Chemical Week, vol. 77 (September 3, 1955), p. 79 f.; J. Bolle, Chimie et Industrie (1960), vol. 83, p. 252.
[40] W. Lange, Berichte der Deutschen Chemischen Gesellschaft (Berlin, 1929), vol. 62, p. 793; vol. 65 (1932), p. 1598.
[41] Gerhard Schrader, U.S. patent 2,336,302 of 1943 (priority in Germany, 1938); S. A. Hall and M. Jacobson, Industrial and Engineering Chemistry (1943), vol. 40, p. 694.
[42] A. M. Mattsen and others, Journal of Agriculture and Food Chemistry (1955), vol. 3, p. 319.
[43] John B. Van Wazer, Phosphorus and its Compounds, 2 vols. (vol. 1, Chemistry; vol. 2 Technology, Biological Functions and Applications), New York: Interscience, 1958, 1961.
U.S. GOVERNMENT PRINTING OFFICE: 1965
For sale by the Superintendent of Documents, U.S. Government Printing Office Washington, D.C. 20402—Price 25 cents
INDEX
Aristotle, 179
Baeyer, Adolf, 193
Bechil, Achild, 179
Berthelot, Marcellin, 189
Berzelius, Jöns Jakob, 182
Black and Bell, plant at Stratford, 182
Boussingault, Jean Baptiste, 185
Boyle, Robert, 178, 179
Brand, H., 178, 179
Buchner, Hans, 197, 200
Calvin, Melvin, 200
Casciarolo, Vicenzo, 179
Chevreul, Michel, 189
Cori, Carl F., 200
Cori, Gerti T., 200
Crookes, William, 192
Davy, Sir Humphry, 185
De Hevesy, George, 198, 200
De la Vega, Garcilaso, 185
De Saussure, Théodore, 185
Euler-Chelpin, Hans von, 197, 200
Fernelius, Jean, 179
Feulgen, Robert, 193
Fischer, Emil, 193
Gahn, Johann Gottlieb, 182
Gay-Lussac, Joseph Louis, 182
Gobley, Nicolas Théodore, 191
Graham, Thomas, 182, 183, 185
Hankwitz, Gottfried, 180
Harden, Arthur, 197, 200
Hartmann, Immanuel Peter, 181
Hellot, Jean, 180
Henry II, King of France, 179
Hittorf, Wilhelm, 181
Hoefer, Ferdinand, 179
Holmberg, Wilhelm, 178
Hoppe-Seyler, Felix, 193
Humboldt, Alexander von, 185
Huygens, Christiaan, 179
Incas, 185
Kletwich, Johann Christopher, 179
Koppe, Émile, 181
Kornberg, Arthur, 200
Kossel, Albrecht, 200
Kraft, Johann Daniel, 179
Kramer, Dr. ——, 181
Kunckel, Johann, 179
Lange, W., 199
Lavoisier, Antoine Laurent, 181, 185
Laws, John Bennet, 186
Leibnitz, Gottfried Wilhelm von, 179
Lennox, Charles, third Duke of Richmond, 185
Leonhardi, Johann Gottfried, 179
Levine, Phoebus Aaron Theodor, 193
Liebig, Justus, 183, 185, 186
Liebreich, Oscar, 191
Lipmann, Fritz, 200
London, E. S., 193
Macquer, Peter Joseph, 180
Marggraf, Andreas Sigismund, 180
Meyerhof, Otto, 194, 200
Miescher, Johann Friedrich, 192
Muspratt, James, 186
Nietzsche, Friedrich, 186, 187, 189
Ochoa, Severo, 200
Pelouze, Théophile Juste, 189
Rouelle, Guillaume François, 181
Scheele, Karl W., 182
Schmiedeberg, Oswald, 193
Schrader, Gerhard, 199
Schrötter, Anton, 181
Stoklasa, Julius, 186
Strecker, Adolf Friedrich, 191
Thudichum, Ludwig, 192
Todd, Lord Alexander, 200
Willm, Edmond, 182
Willstätter, Richard, 191
Wurtz, Adolphe, 185, 191
Transcriber’s Notes
The following typographical errors have been corrected:
Page 180 “Abfällen, Vieweg, Braunschweig,” - had “Viewig”.
Page 188 “wires d from the dynamo D” - had “dynano”.
Page 191 “phosphate are attached, for example,” - had “attached, For”.
Page 192 “But phosphatides occur” - had “phosphatide soccur”.
Page 193 “the nucleic acid from the thymus” - had “nucleidic”.
Page 199 “acetylcholine esterase.” - had “acetylcholin”.
Page 200 “George de Hevesy, Carl F. Cori,” - comma added after Hevesy.
Footnote 39: “See, e.g., Chemical Week, vol. 77” - had “See. e.g.”
Index Entry: “Gahn, Johann Gottlieb, 182” - had “Gähn”
The spelling of “Bertholet” [Claude Louis Berthollet] is as given on the original title page of the work referenced in this paper.
Inconsistent hyphenation of chemical names has been retained.


 Creatine Phosphate
Creatine Phosphate Arginine phosphate
Arginine phosphate